Frederick M. Ausubel, Ph.D.
Massachusetts General Hospital / Harvard Medical SchoolProject Title: Identifying Novel Anti-infectives by High Throughput Screening in Whole Animals
Grant ID: R01-AI-085581
Microbes that cause disease are becoming resistant to antibiotics faster than we can find new ones, making many common infections untreatable and life threatening. Innovative approaches are urgently needed to speed up the discovery of new anti-infectives. This project aims to achieve a paradigm shift in antimicrobial drug discovery by finding next generation anti-infectives that prevent disease by blocking pathogen adaptation to host physiology. Rather than simply preventing bacteria from growing, these new sophisticated drugs will prevent disease by interfering with a microbe's ability to interact with the human body. The findings will help to identify drugs that cure otherwise lethal infections.
Sylvie Breton, Ph.D.
Harvard Medical School / Massachusetts General HospitalProject Title: 3-Dimensional Modeling of Basal Cell Function in Pseudostratified Epithelia
Grant ID: R01-DK-085715
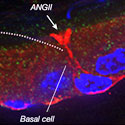
A current paradigm in biology is that basal cells present in the epithelial lining of some organs never come into contact with the inner (luminal) side of the organ. Recently, Dr. Breton and colleagues made a paradigm-shifting discovery that these “so called” basal cells actually extend slender body projections that reach the luminal side of the epithelia and control their function via crosstalk with other epithelial cells. They will take this work one step further and create new model systems to determine the 3-D relationships and functions of different epithelial cell types as the basal cells detect and respond to various drugs, hormones, chemicals and pathogens that appear in the cavity of the organ. A better understanding of how these novel basal cells communicate with adjacent cells will help define disease mechanisms and suggest new diagnostic and therapeutic strategies for male infertility, and diseases of the lung, including asthma, chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF).

Julio A. Camarero, Ph.D.
University of Southern California School of PharmacyProject Title: Cell-Based Screening and Selection of Cyclotide-Based Capture Reagents for Protein Profiling Using Micro-Array Format
Grant ID: R01-GM-090323
High-throughput assays are indispensable for comprehensive functional proteome research. To accelerate research in this field, new protein capture tools for the detection and identification of specific proteins are needed. The reagents must be stable to thermal and proteolytic degradation, have high affinity, be easy to produce and present low cross-reactivity. This proposal presents an innovative approach for screening and selecting a new class of highly stable protein capture reagents and developing a new versatile approach for ligand immobilization that together enable rapid production of cyclotide-based microarrays for proteomics research. These technologies have the potential to accelerate science discovery and to realize the diagnostic and prognostic benefits of clinical proteomics.
Pinchas Cohen, Ph.D.
University of California, Los AngelesProject Title: Novel Mitochondrially Encoded Peptides and Their Role in Health and Disease
Grant ID: R01-GM-090311
Mitochondrial dysfunction has been associated with many diseases, including neurogegeneration, diabetes and cancer, although its exact role in the development of these diseases remains controversial. This proposal tests the paradigm-shifting hypothesis that mitochondrial-derived proteins (MPDs) play a previously unappreciated role in the regulation of cellular and organismal function, and that disregulation of MDPs is important in disease development. Understanding the role of MPDs may lead to development of new therapeutic and diagnostic targets.

Benjamin F. Cravatt, Ph.D. and Thomas Kodadek, Ph.D.
Scripps Research Institute, FloridaProject Title: Massively Parallel Identification of Protein Ligands
Grant ID: R01-GM-090294
This project aims to develop a revolutionary screening platform that will allow for the rapid isolation of hundreds of high affinity and specificity synthetic ligands for proteins in a highly parallel fashion. The identification of large numbers of protein ligands is a high priority for biomedical research. Such ligands could be employed as reagents to construct tools for the discovery of diagnostically useful disease biomarkers. They could also serve as drug leads for a variety of therapeutically interesting targets. Because the ligands will be peptoids, which can be easily synthesized in large quantities by research laboratories lacking specialized organic chemistry skills, the ligands will be more widely accessible to the research community than would be most other classes of protein binding-molecules.
Michael P. Czech, Ph.D.
University of Massachusetts Medical SchoolProject Title: Oral Delivery Vehicles for RNAi Therapies
Grant ID: R01-DK-085753
The use of RNA interference (RNAi) based gene silencing holds great promise as a clinical therapy for many diseases, but such applications face many hurdles including reliable methods for delivery to target tissues and organs. The investigators have developed a novel microparticle technology for oral RNAi delivery to macrophages in living animals and demonstrated in vivo gene silencing and amelioration of inflammation. They now propose to develop this method as a clinical strategy for treating a number of important diseases in which macrophage-mediated inflammation plays a role including type 1 and 2 diabetes, atherosclerosis, arthritis and inflammatory bowel diseases.
Gaudenz Danuser, Ph.D. and Klaus M. Hahn, Ph.D.
Harvard Medical School and University of North Carolina, Chapel HillProject Title: Quantitative Imaging of Signaling Networks
Grant ID: R01-GM-090317
Most of our current understanding of signal transduction pathways relies on solution biochemical analyses of protein interactions and on bulk measurement of select pathways averaged over large cell populations. This is a proposal to establish a new paradigm for the study of cellular signal transduction that combines biosensor design and live cell imaging to produce a transformative approach to studying cellular signal transduction and decision processes. These advances promise a breakthrough in our ability to probe complex, spatially and temporally organized signaling processes in cell models of normal and diseased physiology.

Joshua T. Dubnau, Ph.D.
Cold Spring Harbor LaboratoryProject Title: Genome-Wide Regulatory Network Governing Neuronal mRNA Translation
Grant ID: R01-NS-067690
A fundamental property of the brain is that our experiences cause modifications in the number and strength of the connections among neurons. These changes, which are thought to underlie memory and cognition, require the precise control of the synthesis of specific proteins at the sites of these connections. The mechanisms governing this local synthesis of synaptic proteins are poorly understood, although it is known that disruption of this process has severe consequences. This defect is thought to be responsible for Fragile-X syndrome, the most common inherited form of mental retardation. This proposal describes a method that can provide individual reporters of the regulation of protein synthesis for each gene in the genome, and can identify the regulatory mechanisms controlling each one. The work will be conducted in Drosophila although the high degree of conservation of gene regulatory mechanisms and function, is it likely that much of what we learn will be transferable to humans.
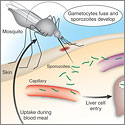
David A. Fidock, Ph.D.
Columbia University Health SciencesProject Title: Exploiting Fatty Acid Metabolism to Cure Malaria
Grant ID: R01-AI-085584
The success of artemisinin-based combination therapies in combating Plasmodium falciparum malaria has inspired calls for the eradication of this devastating parasitic disease. However, new reports of emerging artemisinin resistance, and the absence of alternative first-line drugs, highlight an urgent need for new control methods. This project proposes an entirely novel approach to developing vaccines and drugs to prevent malaria, based on the investigators’ recent discovery of a fundamental difference in the way parasites replicating at the liver versus the blood stage acquire their fatty acids during intracellular growth. Exploiting the dependency of fatty acid synthesis by liver stage parasites, they will develop fatty acid synthesis-deficient, genetically-attenuated parasite vaccines that arrest in the liver, causing a host immune response that protects against infectious challenge. In addition, they will target parasite synthesis as well as salvage of host fatty acids, as a means to develop novel prophylactic and curative medicines that can prevent and control this widespread and devastating disease.
Linda G. Griffith, Ph.D.
Massachusetts Institute of TechnologyProject Title: Perfused 3D Tissue Surrogates for Complex Cell-Cell Communication Systems
Grant ID: R01-EB-010246
A tremendous gap exists between available culture and animal models and methods that can extract detailed information on cell-cell communication networks governing system responses to perturbations, such as an inflammatory cue. Networks inherently operate in a complex, interlinked fashion, and often exhibit non-intuitive outcomes from intervention at a particular point in the network, as evidenced by failure of many targeted therapeutics to operate in the clinical setting after promising results in currently- available preclinical trials. The goal of this project is transform our ability to probe cell-cell communication networks in human cell systems via linking systems biology with tissue engineering. Development of new methods that do not rely on genetic manipulation of the cell populations will generate valuable information about systems operation.

Andrew R. Hoffman, Ph.D.
Palo Alto Institute for Research and EducationProject Title: Role of Long-Range Chromatin Interactions in Genetic Disease
Grant ID: R01-GM-090314
A central paradigm of medical genetics is that the phenotype of a deletion syndrome results from the disruption of the deleted genes themselves. In direct contradiction to this dogma is the idea that genetic diseases can be caused or modified by changes in physical, long-range interactions that normally occur between loci that are now deleted and genes that are far from the deleted region when plotted on a linear genetic map. In some cancers and genetic diseases, long range associations between genes are lost because a part of a chromosome becomes deleted. By examining the network of long range interactions among genes, we will learn how diseases are caused by the change in the expression of many genes which become abnormally regulated when the interactions are lost.
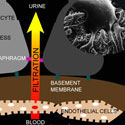
Ravi Iyengar, Ph.D.; John Cijiang He, M.D. Ph.D.; Susana Neves, Ph.D.; James C. Hone Ph.D.; Leslie M. Loew Ph.D.
Mount Sinai School of Medicine | Columbia University | University of Connecticut Health CenterProject Title: Dynamics Underlying Tissue Integrity
Grant ID: R01-DK-087650
This project seeks to address the mechanisms underlying the integrity of tissues, networks of interacting cells and matrices. The project will determine how cells come together to form tissues, and test the hypothesis that tissue integrity results from the integration of information that arises from the dynamic interactions between the different cell types and the matrices that bind these cells together. Using the filtration barrier of the kidney cortex as a model system, the researchers will apply a combination of mathematical models and engineering approaches to develop a 3-D tissue assembly to study this phenomenon. These studies will identify general design principles for assembling functional tissues that can aid in understanding disease processes and for screening for new drugs.
Samie R. Jaffrey, M.D., Ph.D.
Weill Cornell Medical CollegeProject Title: Novel Fluorescent Sensors for Simple, Sensitive, and Specific Protein Detection
Grant ID: R01-EB-010249
The detection of proteins is fundamental to essentially all biomedical research. Current strategies to detect proteins include techniques such as Western blotting and enzyme-linked immunosorbent assays. Currently, there are no techniques that permit the levels of endogenously expressed proteins to be monitored in real time in living cells. This project will address this need by applying a simple, sensitive, and specific method for using novel oligonucleotide- based sensors, that have been developed in the researchers’ lab, for detecting and quantifying proteins. The sensors will be used to monitor protein expression in cells in real time. The experiments will result in powerful new tools for protein detection that will markedly enhance and expand biomedical research.

Ru-Rong Ji, Ph.D., and Charles N. Serhan, Ph.D.
Brigham & Women’s Hospital / Harvard Medical SchoolProject Title: Resolvins, Protectins, and Chronic Pain Resolution
Grant ID: R01-NS-067686
More than 30 million Americans suffer from unrelieved chronic pain, such as nerve injury-induced neuropathic pain. Although a considerable amount is known about how chronic pain is induced, little is known about how acute pain naturally resolves. Current management of chronic pain mainly focuses on two types of drugs, ones that treat pain symptoms by blocking neurotransmission and those that modify disease progression by suppressing neuroinflammation. This project employs a novel approach for chronic pain therapy using newly uncovered endogenous pro-resolving lipid mediators. The project will investigate whether and how these mediators can prevent and reverse neuropathic pain after nerve injury. The results from the proposed studies are likely to transform and positively impact on the entire pain community, from acute postoperative pain to chronic inflammatory and neuropathic pain.
Shohei Koide, Ph.D.
University of ChicagoProject Title: Rational Generation of Directed Protein-Capture Reagents
Grant ID: R01-GM-090324
Protein-capture reagents are indispensable for delineating the molecular mechanisms of diseases, to detect and characterize cellular abnormalities, and to characterize biological effects of drugs. However, the current paucity of high-quality protein-capture reagents presents a major bottleneck in virtually all areas of biomedical sciences. This project will establish a totally new approach to facile generation of detection reagents that are high performance, easy to produce and easily made available to the research community. This innovative and powerful technology will fill a major void in the currently available molecular tools and will have a major impact on virtually all areas of molecular biomedical sciences, diagnosis and drug development.
Calvin Kuo, M.D., Ph.D.
Stanford University School of MedicineProject Title: Three-Dimensional Scaffold-Based Systems for Primary Human Intestinal Culture
Grant ID: R01-DK-085720
The adult human intestine performs essential roles in physiologic homeostasis including digestion, nutrient absorption, secretion of hormones, and immune functions. Diseases of the intestine, such as inflammatory bowel diseases, infection, malabsorptive states and neoplasia, are a considerable source of human morbidity and mortality. Human primary intestinal culture is essentially not utilized as an experimental tool because primary tissue types lack appropriate in vitro culture methods. To meet this need, this project proposes to employ an innovative approach to enable the primary culture of three-dimensional (3-D) human intestinal tissue, which is currently not possible. The ability to reproducibly culture 3-D tissues outside of the body will enable scientists to test new hypotheses and models of physiology and disease, to develop high-throughput screens of pharmaceutical targets, and to enable the expansion of cells for regenerative medicine therapies.
Eric Lagasse, Ph.D.
University of PittsburghProject Title: Organogenesis of Ectopic Tissue in Lymph Node
Grant ID: R01-DK-085711
Hepatocyte transplantation has been reported as a possible therapeutic approach for liver disease; however, transplantation has been directed at the liver itself, limiting efficacy in patients with end-stage liver diseases, when cirrhosis and fibrosis are common. This proposal will demonstrate that the generation of an ectopic liver within lymph nodes is an efficient method to restore hepatic function, highlighting the novel use of this organ as a site for hepatocyte transplantation. The project addresses some of the hurdles confronting the development of complex 3-D tissue models, and provides a new paradigm for tissue modeling, by using lymph nodes as in vivo bioreactors to grow tissue or organ substitutes.
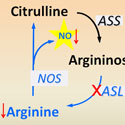
Brendan Lee, M.D., Ph.D.
Baylor College of Medicine / Howard Hughes Medical InstituteProject Title: Argininosuccinate Lyase Is an Essential Regulator of Systemic Nitric Oxide Production
Grant ID: R01-GM-090310
Nitric Oxide (NO) is an essential signaling molecule for diverse physiological and disease processes. While the regulation of NO flux has focused primarily on the study of the three NO synthases (NOS), their respective genetic deficiencies exhibit relatively modest phenotypes, which has led to difficulties in dissecting the specific cellular contributions to NO in different disease processes. This project will study a new way that NO production may be regulated in the body by the chemical argininosuccinate lyase (ASL) which may control the production of arginine needed for NO production. The investigators will determine whether inhibiting ASL is the most effective way for controlling NO production in diseases affecting the brain, heart, and pancreas.
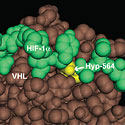
Frank S. Lee, M.D., Ph.D.
University of Pennsylvania School of MedicineProject Title: The Hydroxylprolylproteome
Grant ID: R01-GM-090301
Posttranslational modifications of proteins play important roles in many signal transduction pathways, although the full extent to which these modifications impact cell function is not known. Recent studies have highlighted a key role for a distinctive modification, prolyl hydroxylation, in the hypoxic response. This project seeks to develop novel capture reagents for a posttranslational modification, prolyl hydroxylation, that plays a central role in the oxygen- regulated turnover of Hypoxia Inducible Factor. The long term goal will be to employ these reagents to determine the extent to which prolyl hydroxylation regulates the cellular hypoxic response, and to characterize changes in the hydroxylprolylproteome in response to differing oxygen concentrations. These studies will have implications for understanding diseases such as heart attacks, stroke, and cancer that are characterized by hypoxia.
Kim Lewis, Ph.D.
Northeastern UniversityProject Title: Super-persistent Cells and the Paradox of Untreatable Infections
Grant ID: R01-AI-085585
Infectious disease is often untreatable, even when caused by a pathogen that is not resistant to antibiotics. This paradox could be explained by the fact that microbial populations produce persisters, dormant cells that are not mutants, but phenotypic variants of the wild type that are tolerant to antibiotics. The project will test the hypothesis that the agent responsible for untreatable infections is a super-persister cell which carries a high-persister mutation and has induced stress responses. Mutants of the pathogens are able to enter into a state of dormancy highly tolerant to existing antibiotics. The findings are likely to change the way we view infectious diseases and provide rational approaches for discovering drugs that completely eradicate the infection.
Long-Cheng Li, M.D. and Hao Li, Ph.D.
University Of California, San FranciscoProject Title: Transcriptional Activation by Small RNA: Mechanism and Design Rules
Grant ID: R01-GM-090293
Until recently, small RNAs have been thought to function mainly as the mediators of an evolutionally conserved gene silencing mechanism known as RNAi. This application will establish a new paradigm of gene regulation mediated by small RNA that targets gene promoter sequence to induce potent and prolonged gene activation at the transcriptional level and in a sequence-specific manner, a phenomenon termed RNAa. Despite the enormous potential for reprogramming gene expression in living cells, the molecular mechanism of RNAa is largely unknown and the rules for target selection are still quite obscure. Understanding the mechanism of RNAa will provide new insight to many physiological and diseases processes, may provide a tool to interrogate gene function and study their involvement in diseases, and may be a promising surrogate for traditional gene therapy for many diseases especially cancer.

John T Lis, Ph.D.; Harold Craighead, Ph.D.; Moonsoo Jin, Ph.D.
Cornell UniversityProject Title: Development of High Throughput Aptamer-Based Protein Capture/Detection Assays Towards Comprehensive Analysis of Human Proteome
Grant ID: R01-GM-090320
Novel protein capture and detection reagents are required to understand comprehensively the interplay of the proteome in basic biological processes and in human health and disease. These reagents need to have high affinity and specificity for particular protein targets, and they need to be easily created and produced, and be amenable to modification and immobilization for high throughput analysis of proteins. This project seeks to transform an existing technology to facilitate and streamline identification of novel capture reagents for human proteins and to incorporate these new reagents in assays that can accommodate simultaneous analysis of many proteins. This project will not only develop new technology and assays that enable analysis of human proteins critical in human health and disease, but also generate the novel reagents that can be used for therapy.
Jaroslaw P. Maciejewski, M.D., Ph.D.
Cleveland ClinicProject Title: Viral Pathogenesis of Idiopathic Bone Marrow Failure and Immune Cytopenias
Grant ID: R01-AI-085578
Many diseases strike humans without obvious cause and, despite intense research, their etiology remains idiopathic. For example, it is likely that some idiopathic or autoimmune conditions or even cancers may be attributable to infectious agents even if conventional research avenues favor other mechanisms. The proposal will test the hypothesis that viral pathogens constitute the inciting events in aplastic anemia or other immune-mediated cytopenias, challenging the traditional understanding of autoimmunity in these specific conditions. Contesting current theories of pathogenesis for such conditions may result in unexpected progress and new lines of scientific inquiry. If infectious agents, likely viruses, can be identified, such a discovery would change the paradigm not only for these diseases but also for many other autoimmune diseases, and open avenues to prevention, diagnostics and effective treatments.
Peter A. Margolis, Ph.D. and Michael Seid, Ph.D.
Cincinnati Children's Hospital Medical CenterProject Title: Open Source Science: Transforming Chronic Illness Care
Grant ID: R01-DK-085719
Chronic illness kills too many and costs too much. Reducing this burden is possible, yet within our current health care system, Americans receive about 50% of indicated care and patients follow doctors' orders about as often. The current chronic illness system needs to be changed in order to deliver better care. This project will join patients and physicians together in a collaborative innovation network to design, prototype and optimize, and evaluate a system for improving the chronic illness care system. If successful, this project will lead to improved care and self- management, better outcomes for people with chronic illness, and lower costs of care.
David M. Markovitz, M.D.
University of Michigan at Ann ArborProject Title: Replication of Human Endogenous Retroviruses in Modern Humans
Grant ID: R01-AI-144043
The functions of the approximately 98% of the human genome that do not encode human cellular proteins remain to be elucidated. Actively replicating endogenous retroviruses entered the human genome millions of years ago and became a stable part of the inherited genetic material, with retroviral elements presently making up approximately 8% of the modern human genome. This project will test the hypothesis that endogenous retroviruses, such as modern HERV-K (HML-2), can still replicate in modern humans using cutting-edge and complementary techniques. Proving this hypothesis will be contrary to the entrenched dogma and have substantial implications for human genetics, cancer biology, and the safety of the human blood supply.

Sanford Markowitz, M.D., Ph.D.
Case Western Reserve University School of MedicineProject Title: Identifying Inborn Genetic Susceptibility to Development of Cancer Metastasis
Grant ID: R01-CA-144040
One third of all individuals who develop colon cancer will die of metastatic spread of this disease. The classic genetic model for colon cancer development holds that colon cancer progression is due to accumulation of multiple gene mutations in the cancer cell. This proposal advances the novel paradigm that cancer metastasis, the ultimate cause of death from this dread disease, is not due to new "metastasis-causing" gene mutations in the cancers, but rather is crucially depend on inborn genetic susceptibility factors. The import of this new paradigm for cancer metastasis will be felt in the identification of entirely new biological pathways that will be key to cancer management, to cancer prognosis, and to developing new and more effective anti-cancer therapies.
Wallace Marshall, Ph.D.
University of California, San FranciscoProject Title: Pattern Formation and Regeneration in a Single Cell
Grant ID: R01-GM-090305
The ability of cells to form defined shapes, and to dynamically regenerate their cytoskeletal and membrane structures following damage, is one of the current mysteries of cell biology. Most studies of regenerative medicine have focused on developing methods to use stem cells to replace damaged cells. This proposal adopts an alternative approach using a classical model system, the giant ciliate Stentor, to study how injured cells repair themselves. In addition to being a fundamental cell biological problem, understanding development and regeneration of cellular morphology would be a starting point for developing a whole new approach to regenerative medicine, in which rather than attempting to replace damaged cells with stem cell derived substitutes, one would induce the damaged cells to regenerate in situ.
Steven McKnight, Ph.D. and Andrew Pieper, M.D., Ph.D.
UT Southwestern Medical Center / The University of IowaProject Title: Discovery, Characterization and Preclinical Development of Pro-Neurogenic Drugs
Grant ID: R01-MH-087986
Partha P. Mitra, Ph.D.
Cold Spring Harbor LaboratoryProject Title: The Missing Circuit: The First Brainwide Connectivity Map for Mouse
Grant ID: R01-MH-087988
Brain function is dictated by its circuitry, yet we know little about its wiring architecture: in the most-studied mammal (rat), only an estimated 10–30% of the long range circuit connections have been probed. The study of mouse models of neuropsychiatric disorders provides hope for the development of therapies for these burdensome illnesses, but progress has been slow due to the lack of knowledge about how the mouse brain is wired. This project aims to close this gap by generating the first brain-wide wiring diagram of mouse, using automating techniques that are known to work but are labor-intensive. If successful, the project has the potential to fundamentally transform our understanding of brain function and brain disorders.
Deborah G. Murdock, Ph.D.
Vanderbilt UniversityProject Title: Fatty Acid Signals as Quorum Sensing Regulators of Mitochondrial Biogenesis
Grant ID: R01-GM-0900308
Mitochondrial number is very tightly regulated within the cell. Dysregulation of mitochondrial metabolism leads to decreased energy production, increased reactive oxygen species (ROS), and altered apoptosis. These three factors play a role in a multitude of diseases, including heart disease, diabetes, cancer, obesity, and neurodegenerative diseases such as Alzheimer's and Parkinson's diseases. This proposal will investigate the phenomenon of nuclear/mitochondrial communication and its role in regulation of mitochondrial number. The project will investigate the way in which cells regulate the amount of mitochondria within a cell, with an eye towards their evolutionary origins.

Sheila T. Murphy, Ph.D.; Lourdes Baezconde Garbanati, Ph.D., M.P.H.; Sandra Ball-Rokeach, Ph.D., and Robert Haile, Ph.D.
Annenberg School for Communication | Keck School of Medicine | University of Southern CaliforniaProject Title: Transforming Cancer Knowledge, Attitudes and Behavior Through Narrative
Grant ID: R01-CA-144052
The proposed research challenges the assumption that a traditional straightforward recitation of the facts is the optimal way to convey health-related information and empirically tests whether utilizing a narrative format might produce a greater and longer lasting impact on knowledge, attitudes and prevention behavior. It also involves examination of the effect of communication modality (i.e., print, audio, audiovisual, interactive) on attention, behavior change, and retention of cancer-relevant information. Although the proposed research will focus on breast and cervical cancer, the results have clear implications for virtually all health care communication and could radically change how health messages are conveyed across different ethnic groups, generations and modalities.

Kevin Niswender, M.D., Ph.D. and Aurelio Galli, Ph.D.
Tennessee Valley Healthcare System and Vanderbilt University School of Medicine | Vanderbilt University School of MedicineProject Title: Insulin Regulation of Monoamine Signaling: Pathway to Obesity
Grant ID: R01-DK-085712
Obesity and other dopamine-related pathologies such as schizophrenia, bipolar disorder, and attention-deficit disorder are a tremendous public health burden. Despite knowing better, we consume too many calories, too much fat, and too much sugar. This proposal promises to transform our approach to this problem of obesity by analyzing how parallel changes in insulin and dopamine signaling, induced by high-fat feeding, cement changes in feeding behavior that lead to obesity and related co-morbidities. The finding may allow the development of new therapeutic interventions.
Julie Parsonnet, M.D.
Stanford University School of MedicineProject Title: Childhood Infection and Prevention of Obesity
Grant ID: R01-HD-063142
There has been a 4-fold increase in obesity U.S. children over the last 40 years. The dramatic rise in overweight and obesity over the last 40 years coincides with equally dramatic decreases in childhood infections. Infections of childhood are disappearing due to changes in family size, improvements in hygiene, the addition of new vaccines, and other preventive health measures. This proposal seeks to determine whether the inverse trends of rising obesity and declining infection are coincidently related or causal. This study intends to establish methods and collect preliminary data to determine whether decreases in childhood infection are linked to development of obesity.

Gerald H. Pollack, Ph.D.
University of WashingtonProject Title: Unexpectedly Profound Role of Water in Biology and Medicine
Grant ID: R01-GM-093842
Although water's role in biological function is considered secondary, recent evidence from this principal investigator’s laboratory implies that its role may be more primary. This project will test whether water plays a primary role in a number of representative biological systems, including osmosis and diffusion, surface tension and oxygen exchange, self-assembly, light-induced effects, vascular effects, and global health applications. If the newly discovered features prove to be centrally relevant as hypothesized, then their relevance might extend more generally. If so, then this water-based approach may crack open the door to a new realm of biological understanding.
Charles M. Rice, Ph.D. and Sangeeta Bhatia, Ph.D.
Rockefeller University | Massachusetts Institute of TechnologyProject Title: Modeling Human Hepatotropic Infections in Complex Tissue Organoids
Grant ID: R01-DK-085713
The human liver serves as the reservoir for several important human pathogens, including hepatitis B (HBV) and C viruses (HCV) and Plasmodium species, all of which represent serious global health concerns. A vaccine for HCV has not yet been developed, and, while HCV-specific protease and polymerase inhibitors are showing promise in early clinical development, rapid emergence of resistance indicates that additional targets and combinations of antivirals will be needed for effective control. The scarcity of in vitro and in vivo systems that faithfully mimic liver biology and susceptibility to human hepatotropic pathogens has severely hampered drug and vaccine development. This project uses an interdisciplinary approach that combines tissue engineering with molecular virology and humanized mouse technology to create platforms that will facilitate studies of basic virus-host and virus-virus interactions, promote understanding of the mechanisms of liver disease progression, and provide predictive systems to test drug and vaccine efficacy and toxicity.
Richard W. Roberts, Ph.D. and Hyongsok (Tom) Soh, Ph.D.
University of Southern California | University of California, Santa BarbaraProject Title: Polypeptide Design with Proteomic Scope Via Microfluidic mRNA Display
Grant ID: R01-AI-085583
In this project, the investigators propose to combine mRNA display (a protein design/evolution method) and high efficiency microfluidic sorting to create a new technology-microfluidic mRNA display-for the purpose of enabling design of peptides and proteins that can be used as protein capture reagents. They will develop and apply this powerful new technology toward creating a comprehensive reagent set aimed at the Hepatitis C virus (HCV) proteome. Applying high- throughput approaches to create new protein capture reagents has the potential to speed the pace of proteomics research.
Bela Suki, Ph.D.
Boston UniversityProject Title: Regulatory Roles of Variable Mechanical Stimuli in Cell Function
Grant ID: R01-HL-098976
The basic functions of many cell types are sensitive to physiological levels of mechanical forces acting on them and this phenomenon is always studied in the laboratory using monotonous mechanical stimuli. However, cells in the body are exposed to irregularly varying stimuli and this variability may fundamentally alter all essential cell functions including secretion, growth and death. Uncovering how cells deal with such physiological variability may help understand how cells work in real living tissues as well as the pathogenesis of several major diseases including atherosclerosis, neuro-degenerative diseases, metabolic disorders, aging or cancer.
Muneesh Tewari, Ph.D.
Fred Hutchinson Cancer Research CenterProject Title: RNA as a Hormone: Systemic Signaling in Mammals Via Circulating, Cell-Free Small
Grant ID: R01-DK-085714
The conventional paradigm of the endocrine system does not include RNA molecules as a class of hormones. This proposal seeks to establish the new scientific paradigm that RNA molecules can function as hormones, traveling via the bloodstream to target organs where they are taken up and influence the activity of specific recipient cells. To establish this new paradigm, the researchers will focus on microRNAs secreted into the blood by cancer cells and use mouse models to determine whether the microRNAs are taken up by and influence distant organs and tissues. Establishing this new paradigm will have a major impact not only on basic understanding of human physiology but could also open up new ways of diagnosing and treating disease based on RNA hormones in the blood.
Loren D. Walensky, M.D., Ph.D.
Dana-Farber Cancer InstituteProject Title: A Lexicon of Stapled Peptide Helices Engineered to Capture the Protein Interactome
Grant ID: R01-GM-090299
Whether fleeting or stable, normal or aberrant, protein interactions and their sites of contact form the basis for discovery of biological pathways, disease mechanisms, and opportunities for therapeutic intervention. Like the teeth of a key that perfectly fit into a lock, complementary protein shape is critical to the execution of biological interactions. The goal of this proposal is to intertwine chemistry, biology, and medicine to create a transformative high- throughput technology that precisely identifies protein targets and their explicit sites of interaction. This new technology will address the current formidable challenge of identifying, distinguishing, and drugging the broad array of human protein targets.
Robert B. Wilson, M.D., Ph.D.
University of Pennsylvania School of MedicineProject Title: Random shRNA Selection
Grant ID: R01-GM-090304
The existing paradigm for the use of RNA interference (RNAi) to develop small-RNA therapeutics and biologic tools is to interfere with the expression of a single gene using a short-hairpin-loop RNA (shRNA) or short-interfering RNA (siRNA). Not surprisingly, small-RNA therapeutics and biologic tools based on single gene interference are often plagued by "off-target" effects, resulting in poor therapeutic indices. This project challenges the “single gene paradigm” and proposes to develop small-RNA therapeutics and biologic tools using a novel high throughput approach for rapidly and simultaneously screening thousands of random shRNAs against a shRNA-expressing library that is completely random at the nucleotide level. The approach holds promise for identifying and optimizing shRNA sequences for studies of stem-cell induction and cell differentiation, and for developing small RNAs to be used as therapeutics or biologic tools.
Joseph C. Wu, M.D. Ph.D.
Stanford University School of MedicineProject Title: Re-Education of the Immune System for hES Cell Tolerance
Grant ID: R01-AI-085575
Before human embryonic stem (hES) cell-based therapies for conditions such as neuronal disease, cardiovascular disease, diabetes, Parkinson's, and others can be translated to the clinic, new strategies are needed to overcome the immunological barrier to usage. To address this critical bottleneck, this interdisciplinary team of investigators will develop novel and effective strategies to induce immunological tolerance for hES cell therapy. The goal is to “re-educate" the immune system towards achieving tolerance of hES cell-based therapies by combining hematopoietic chimerism and thymic regeneration. If successful, the project will represent a major scientific advancement in hES cell immunology and a significant step toward their eventual clinical translation.
Xiaoliang S. Xie, Ph.D.
Harvard UniversityProject Title: Imaging the Invisible with No Labels: A Major Opportunity in Biology and Medicine
Grant ID: R01-EB-010244
Small molecules such as metabolites and drugs are crucial to the biochemistry of living organisms but are difficult to study because they cannot be visualized easily in a living cell or organism. Label-free optical imaging based on Raman scattering is a highly desirable approach to use, but is limited by low sensitivity and long acquisition times. This project team recently developed stimulated Raman scattering (SRS) microscopy which offers an unprecedented combination of high sensitivity, rapid image acquisition, chemical specificity and noninvasiveness. Studies of lipid metabolism and drug distributions in tissue are already underway in their laboratory. In this TR01 project, they will continue to probe lipid metabolism, including lipogenesis, lipolysis and insulin resistance. The ability to visualize small molecules such as metabolites and drugs in living cells and organisms without labels will revolutionize biomedical research, particularly lipid metabolism, pharmacokinetics and cancer diagnosis.
Mark J. Zylka, Ph.D.
University of North Carolina at Chapel HillProject Title: Harnessing Ectonucleotidases to Treat Chronic Pain
Grant ID: R01-NS-067688
More Americans suffer from chronic pain than heart disease, diabetes and cancer combined. Unfortunately, existing analgesics are not completely effective for all pain conditions and have serious side effects. A critical challenge for modern biomedical research is the need to provide pain relief without serious side effects. This project addresses this need by harnessing enzymes, called ectonucleotidases, that are found in nociceptive (pain-sensing) circuits in nervous system dorsal root ganglia (DRG) and in the spinal cord. Ectonucleotidases degrade purine nucleotides (like ATP and ADP) that cause pain into adenosine-a compound that has analgesic properties in rodents and humans. These studies are important in developing new proteins and small molecules that target ectonucleotidases for the treatment of acute and chronic pain, and have the potential to transform how we treat pain in millions of patients with fewer side effects.



