After Submission
Disclaimer:
The information in these pages is meant to provide general guidance. Instructions and procedures outlined in the funding opportunity, SF424 Application Guide, and NIH Grants Policy Statement take precedence over any information provided and should be referred to for complete and comprehensive directions.
There's still more to do after you push the submit button. Learn more about what happens after your application is submitted, including information on:
Post-submission materials are those submitted after submission of the grant application but prior to initial peer review. They are not intended to correct oversights or errors discovered after submission of the application, but rather allow applicants the opportunity to respond to unforeseen events. See NOT-OD-19-083 for more details. For this NOFO, only documents to inform of unforeseen events, such as natural disasters, that substantially affect the ability to execute the proposed research will be accepted.
NIH seeks the highest level of ethical standards for peer review. NIH policy is intended to promote a bias-free process that evaluates grant applications in a fair, equitable, and timely manner. Peer review is conducted at two levels as mandated by statute and federal regulation. All review criteria and considerations are specified in the funding opportunity.
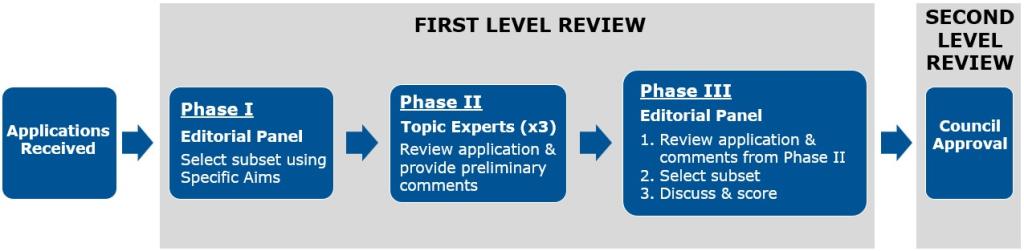
The first level of review for the Transformative Research Award is conducted in three phases in a Special Emphasis Panel that will review all Transformative Research Award applications. The process is overseen by a Scientific Review Officer (SRO) from the Center for Scientific Review (CSR). All applications are automatically sent to the Special Emphasis Panel, and reviewers change from year to year.
The second level of review is performed by the NIH Office of the Director's national advisory council called the Council of Councils. The council is composed of both scientific and public representatives chosen for their expertise, interest, or activity in matters related to health and disease. Applications must receive approval at both levels of review to be eligible for funding.
Below is a diagram of the Transformative Research Award review process.
Role of the Scientific Review Officer
The Scientific Review Officer (SRO) is responsible for ensuring that each application receives an objective and fair initial peer review, and that all applicable laws, regulations, and policies are followed. The duties of the SRO include:
- Analyzing the content of each application and checking for completeness
- Documenting and managing conflicts of interest
- Recruiting qualified reviewers based on scientific and technical qualifications and other considerations, including:
- Authority in their scientific field
- Dedication to high quality, fair, and objective reviews
- Ability to work collegially in a group setting
- Experience in research grant review
- Balanced representation
- Assigning applications to reviewers for critique preparation and assignment of individual criterion scores
- Attending and overseeing administrative and regulatory aspects of peer review meetings
- Preparing summary statements for all applications reviewed
The SRO is the point of contact for all review-related questions and issues.
First Level of Review
The Transformative Research Award differs from traditional NIH reviews in that the first level of review occurs in three phases by two separate groups of reviewers with different perspectives. Applications are reviewed by a broad thinking editorial panel in Phases I and III and by topic experts ("mail" reviewers) in Phase II.
The Transformative Research Award is designed to support exceptionally innovative research projects with the potential to have a profound effect on an area of research relevant to the broad mission of NIH. The innovation may be technical, conceptual, or (often) a combination of both. Given the high level of innovation expected, conventionally detailed experimental plans and extensive preliminary data are not required. Accordingly, reviewers will emphasize the strength of the conceptual framework, the level of innovation, and the potential to significantly advance our understanding or capability in a field relevant to NIH.
All standard NIH review criteria are used to evaluate applications (some are listed below), but emphases will be on innovation and broad impact of the research.
- Significance of the problem
- Qualifications of the investigator
- Innovation of the approach
- Strength of the approach
- Environmental support and resources
The Transformative Research Award will use the Simplified Review Framework for review. The Simplified Review Framework retains the five criteria but reorganizes them into three factors — two receive numerical criterion scores and one is evaluated for sufficiency. All three factors are considered in arriving at the Overall Impact score. The reframing of the criteria serves to focus reviewers on three central questions for evaluation: How important is the proposed research, how rigorous and feasible are the methods, and whether the investigators and institution have the expertise/resources necessary to carry out the project.
- Factor 1: Importance of the Research (Significance, Innovation), scored 1-9
- Factor 2: Rigor and Feasibility (Approach), scored 1-9
- Factor 3: Expertise and Resources (Investigator, Environment), to be evaluated as either sufficient for the proposed research or not (in which case reviewers must provide an explanation)
The change to having peer reviewers assess the adequacy of investigator expertise and institutional resources as a binary choice is designed to have reviewers evaluate Investigator and Environment with respect to the work proposed. It is intended to reduce the potential for general scientific reputation to have an undue influence.
Full review criteria are listed in the funding opportunity.
Phase I
Phase I is conducted by an editorial panel composed of scientists from an array of scientific backgrounds. The composition of the editorial panel attempts to capture a wide breadth of scientific expertise, experiences, and perspectives.
The editorial panel uses the Specific Aims pages to identify a subset of applications with the most transformative potential. The review process ends for the unselected applications, while the subset of selected applications moves on to Phase II.
Phase II
Phase II is conducted by topic experts (also called "mail" reviewers) who are knowledgeable in the application’s subject area and are assigned based on close matching of their expertise to the application topic. Each application will have three topic experts assigned to review it. Each expert will provide comments, which are provided to the editorial panel for Phase III.
Phase III
In Phase III, the editorial panel, informed by the critiques from the topic experts, will select a further subset of applications to be discussed and scored by the panel. All other applications will be designated as "not discussed." Typically, only about 18-20% of the applications are chosen for discussion. Panel members provide preliminary scores and lead a panel discussion of their assigned applications. Following the panel discussion, the applications are scored by the whole panel. All review criteria will be assessed in the scoring by the panel. The panel discussion is captured by the SRO and is summarized in the summary statement.
Scoring & Summary Statement
Each panel member privately scores each discussed application. A raw score of 1 is the best, while 9 is the worst. The SRO collects and averages all the panel scores and multiplies the resulting number by 10 to yield an overall impact score. A discussed application can have an overall impact score of 10 (best) to 90 (worst). The panel has access to the full range of scores to provide discrimination among applications within this select subset. These scores should not be compared to a regular R01 study section review.
After the meeting, all discussed applications will receive an overall impact score within three business days through the PD/PI's eRA Commons account. The overall impact score indicates the reviewers' judgment of the significance of the problem, transformative potential of the project, and the innovativeness of the approach. There is a correlation between a strong impact score and funding. However, there is no strict cutoff or pay line for funding. And because applications are responding to a Request for Applications (RFA), the scores are not percentiled, which makes their interpretation difficult. NIH staff cannot disclose where an impact score falls relative to other application scores.
A summary statement prepared by the SRO will be available within 30 days of the review through the PD/PI's eRA Commons account. The summary statement of discussed applications includes critiques from the Phase II topic experts and a brief summary of the panel discussion. The information provided in the summary statement is valuable and provides critical feedback. However, it is not intended to be an exhaustive critique and will not contain every point reviewers found to be problematic. Applications that made it past Phase I are considered "not discussed" and are given a summary statement with critiques from the mail reviewers. Applications that did not make it through Phase I are given a description of the review process.
After Receiving the Summary Statement
If you have any questions about your Summary Statement, you should reach out to the scientific contact for the Transformative Research Award (listed in the funding opportunity). The scientific contact attends the review and may be able to provide more insight into the panel discussion and help clarify some of the comments. You can also ask about the probability of funding and get advice on what to do if your application is outside of the likely pay range. Contacting NIH staff to “sell” your application or to express differences in scientific opinion related to the reviewers’ comments will not affect the likelihood of funding.
It is best to contact NIH staff by email to schedule a time for a meeting. That gives staff time to read your summary statement and review any notes. And be patient. They will receive numerous inquiries and may not be able to respond to yours immediately.
PD/PIs of discussed applications are given an opportunity to submit an optional two-page response to their summary statement. The response should address issues and concerns brought up by reviewers, but the response will not be seen by reviewers and is not a part of the review. The response remains an internal NIH document used only by NIH staff during funding deliberations. The scientific contact will reach out to eligible applicants directly with more information on the response and the deadline.
Second Level of Review
The Council of Councils performs the second level of review for the Transformative Research Award and assesses the first level of review for fairness and uniformity in the application of review criteria. It is meant to ensure the initial review was conducted with the appropriate expertise, procedures, and without conflicts of interest. The Council of Councils is not tasked with reviewing the applications for scientific or technical merit and is not asked to provide recommendations on which applications should be funded. The Council of Councils votes en bloc for concurrence with the first level review recommendations. Applications must receive approval from the council to be eligible for funding.
Roles & Responsibilities
Timely and effective communication between the award recipient and NIH staff is critical throughout pre-award, award, and post award processes. At this stage the following people will work closely together:
NIH Staff
- Grants Management Officer (GMO): The GMO signs the Notice of Award (NoA) and is the NIH official responsible for the business management and other non-programmatic aspects of the award. GMOs ensure that laws, regulations, and administrative policies are followed.
- Grants Management Specialist (GMS): The GMS works with the GMO on the day-to-day management of the grant. The name and contact information of the GMS assigned to a particular grant appears on the NoA.
- Program Official (PO): The PO is responsible for the programmatic, scientific, and/or technical aspects of assigned applications and grants. The PO coordinates with grants management on post-award administration.
Recipient Organization Participants
- Authorized Organizational Representative (AOR): The AOR, also known as Signing Official (SO) in the eRA Commons, is the designated representative of the recipient organization in matters related to the award and its administration. Their signature certifies that the applicant organization will comply and is accountable for all assurances and certifications referenced in the application. This individual's signature further certifies that the applicant organization will be accountable both for the appropriate use of funds awarded and for the performance of the grant-supported project or activities resulting from the application.
- Program Director/ Principal Investigator (PD/PI): The PD/PI is the individual designated by the applicant organization to have the appropriate level of authority and responsibility to direct the project or program supported by the award. The PI is responsible and accountable to the recipient organization for the proper conduct of the project or program, including the submission of all required reports.
The PD/PI is the core member of the award recipient team responsible for ensuring compliance with the financial and administrative aspects of the award. The PD/PI work closely within the award recipient organization to create and maintain necessary documentation, such as technical and administrative reports, preparing justifications, appropriately acknowledging federal support of research findings in publications, announcements, news programs, and other media, and ensuring compliance with other federal and organizational requirements.
NIH encourages the PD/PI to maintain contact with the NIH program officer with respect to the scientific aspects of the project and the grants management officer concerning the business and administrative aspects of the award.
Just-in-Time Request
Some important time-sensitive information isn’t included in your application. Instead, you prepare it separately and send it in before awarding in a process called Just-in-Time (JIT).
After initial peer review, NIH sends an automatic email requesting JIT information for applications within a competitive funding range. The notification is NOT a Notice of Award, nor should it be construed as an indicator of possible award.
Requested JIT information includes:
- Other support
- Certification of Institutional Review Board (IRB) approval
- Certification of Institutional Animal Care and Use Committee (IACUC) approval
- Human subjects training certification for all key personnel
JIT information must be submitted for NIH review and evaluation prior to making an award. This information may be submitted via the Just-In-Time function within eRA Commons. If you have questions about the JIT process, contact the Grants Management Specialist assigned to your grant.
Funding Deliberations
Following review of all applicable information, the NIH Office of the Director and the High-Risk, High-Reward Research program working group (composed of NIH staff from other NIH institutes and centers) will determine whether an award will be made.
The score given to an application during the initial peer review process is an important factor and best indicator of likely funding but is not the sole factor used in making funding decisions. The following are considered in making funding decisions:
- Scientific and technical merit of the proposed project as determined by scientific peer review with consideration of the PD/PI’s optional summary statement response
- Availability of funds
- Conformance to clinical trial research policies of the administering Institute or Center
- Relevance of programmatic priorities, including:
- Despite inherent scientific and technical risks, the potential for the research to result in scientific breakthroughs of broad impact
- Unusually cross-cutting science.
- Scientific balance in the portfolio of Transformative Research Award-supported research
- Potential to invigorate exceptionally innovative and impactful science broadly across the nation
The NIH Office of the Director funds the majority of awards. However, other NIH Institutes and Centers are invited to fund Transformative Research Award applications that they deem meritorious and that fit within their mission and priorities. The NIH Office of the Director does not play a role in the funding deliberations of the Institutes and Centers, but the process is managed by the institute/center’s member of the High-Risk, High-Reward Research program working group.
Pre-Award Negotiation
The pre-award process involves significant communication between the NIH and the applicant organization and includes negotiation if significant adjustments are required prior to award. Some of the issues NIH staff will be considering during award negotiations include:
- Initial peer review recommendations- Peer reviewers may recommend changes to the specific aims. These recommendations are provided in the summary statement. Under these circumstances, NIH staff will include these recommendations in consideration of a potential award.
- Overlap — Program and grants management staff will review the other support information to ensure there is no overlap with already funded projects (e.g., support, commitment, or budgetary).
- Level of effort — Program and grants management staff will ensure sufficient levels of effort are committed to support the approved project.
- Facilities and Administrative (F&A) Costs — Grants management staff will utilize the negotiated F&A costs (also known as indirect costs) for each grant. More information on the reimbursement of F&A costs can be found in the NIH Grants Policy Statement.
Notice of Award
You may receive communications from NIH staff regarding the intent to make an award, but nothing is fficial until you receive the Notice of Award (NoA).
The NoA is the legal document issued to notify the award recipient that an award has been made and that funds are now available for the project. The NoA includes the terms and conditions of the award, your project's start and end dates, and how much money you will receive for current and future years. It also provides contact information for the assigned program officer and grants management specialist. It is important that you read and understand your NoA.
NoAs are issued annually for each budget period and are contingent upon annual assessment of research progress and the availability of funds.
More questions? Contact us at [email protected].



