SPARC2 (SP2)
Next Generation Tools and Technologies
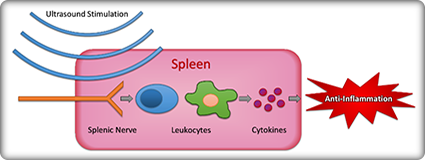
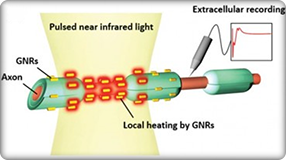
SPARC2 supports the development of tools and technologies to facilitate the progress of other SPARC components, particularly SPARC1. The scope encompasses a wide range of capabilities, spanning the fields of photonics, systems engineering, virology and genomics, device design and manufacture, surface chemistry, tissue engineering, neural interfacing, biomarker sensing, and more.
It is expected that SPARC2 teams will make promising next-generation mapping technologies freely available and that other SPARC teams will adopt these technologies into their research plans. Moreover, documentation, data, and training pertaining to these technologies will be available through the efforts of SPARC4. Ultimately, the knowledge produced by the SPARC program – the comprehensive maps and new insights into translation from animal models to human patients – is expected to inform the next generation of neuromodulation therapies.
View SPARC2 funded research here.