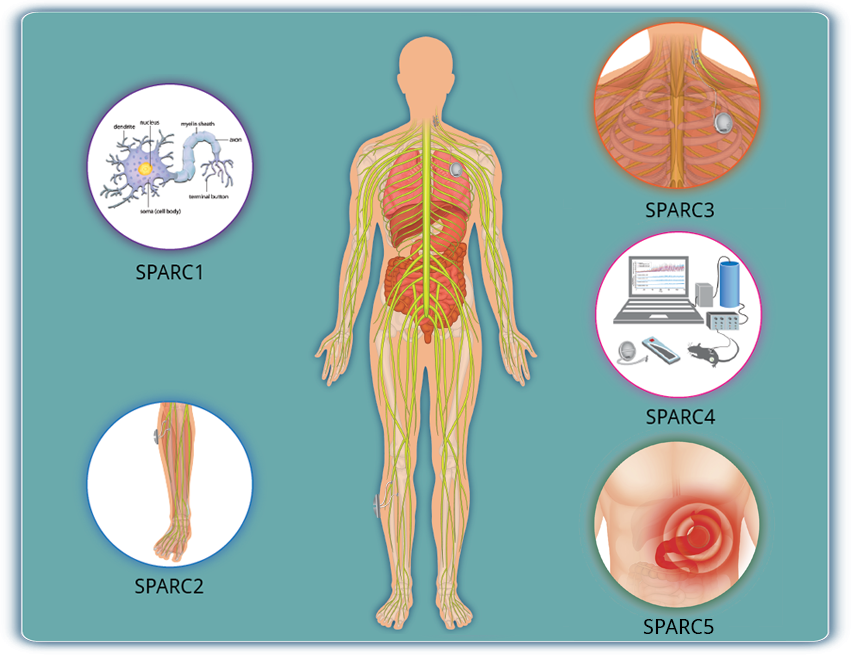
SPARC1 (SP1)
Anatomical and Functional Mapping of the Innervation of Major Internal Organs
SPARC1 supports studies in animal models and humans, including cadaveric tissue, that create new anatomical and physiological data sets to generate and address hypotheses in the following areas:
- coursing and branching of nerves and the distribution of axon terminals;
- the structure of nerve-organ synapses;
- the cross-sectional organization of nerves;
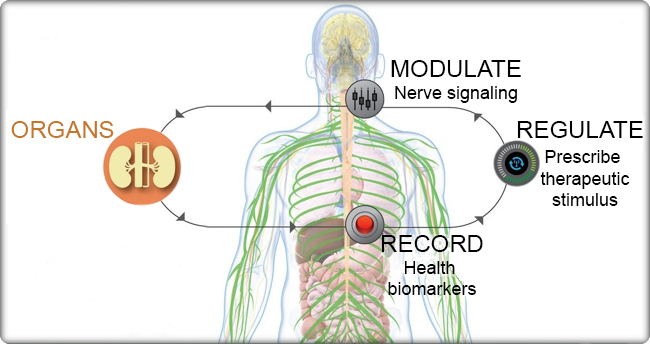
- the effect of firing patterns on organ function;
- the functional relationships between neural signals and end-organ responses;
- the variability in expression/anatomical representation of the neural cell-types at each potential point of implantation of neuromodulation interfaces;
- differences in PNS neuroanatomy and control of organ activity between animal models and humans;
- translating animal data to human applications;
- the variance in effects and side effects between individuals (e.g., inter-individual variability in anatomy and response).
View SPARC1 funded research here.