When scientists want to simplify things, they sometimes refer to the human body as “giant bags of mostly water.” The human body is a complex collection of fluids that must be carefully balanced so that internal processes can happen, and the person can remain healthy. To maintain the balance of fluids, nutrients, and other molecules within the body, we rely on our kidneys to regulate filtration, absorption, and blood pressure. While scientists know the general anatomy and physiology of the kidney, the processes at the microscopic level remains less well known.
To address some of these knowledge gaps, NIH Common Fund Human BioMolecular Atlas Program (HuBMAP) funded researchers led by Dr. Sanjay Jain at Washington University at St. Louis and Dr. Gloria Pryhuber at the University of Rochester Medical Center used cutting-edge tissue processing and light sheet fluorescence microscopy (LSFM) methods to gain new insights about the changes in structure, function, and physiology the kidney undergoes during a lifetime. Their findings in Nature Communications, entitled Three dimensional multiscalar neurovascular nephron connectivity map of the human kidney across the lifespan, delves into their techniques, including how they examined samples from human patients of many different ages.
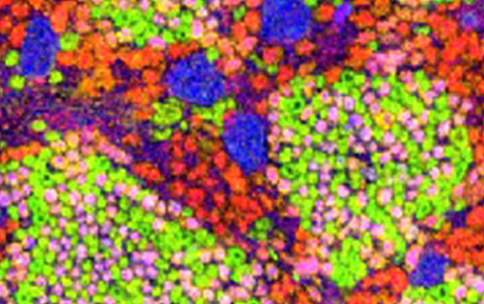
LSFM is an advanced fluorescent microscopy technique that allows researchers to see inside the kidney at subcellular levels and reconstruct what the organ looks like. Researchers use LSFM to send a thin sheet of light through the issue sample and then collects the differently colored fluorescent signals from that plane into one two-dimensional image. The researchers scan individual sections of the entire tissue block from many planes to collect these two-dimensional images. The images are then combined to reconstruct a three-dimensional image of the sample, revealing details of the kidney’s internal structure at the subcellular level.
Kidneys are composed of millions of nephrons, which are the functional units responsible for filtering blood. At the opening of each nephron, there is a network of blood vessels called the “glomerulus.” The researchers found that glomeruli organize themselves into interconnected communities through nerve cells that increase in density as the kidney develops throughout a person’s lifetime. Most importantly, the researchers found several patterns of connected communities that they named “mother-gloms.” The researchers believe the mother-gloms provide structural organization to sense or relay signals throughout the nephron. By doing this, the mother-gloms seem to coordinate physiological responses by decreasing the burden on individual nephrons. This finding is just one of the many insights into kidney function throughout development, life span, and health status.
Using these new and innovative techniques, the researchers discovered changes within the kidney that affect its structure and function. They also found an external network of nerve cells that organize the regions of the kidney into “mother glomeruli”, which serve as control centers throughout the organ. These control centers appear to help synchronize the responses to changes in the fluid balance of the body.
This work explains how the structure of the kidney enables coordinated responses across the organ and how disruptions in this system can lead to diseases like diabetes and other kidney conditions. It also reveals how kidney physiology adapts and changes over a person’s lifetime, offering crucial insights into both the prevention and management of kidney health and related illnesses. This finding exemplifies how development of innovative techniques and methods can lead to greater discoveries and treatments for human health.
Three dimensional multiscalar neurovascular nephron connectivity map of the human kidney across the lifespan. Nat Commun. 2025 Jun 3;16(1):5161. doi: 10.1038/s41467-025-60435-8. PMID: 40461472. PMCID: PMC12134253. DOI:10.1038/s41467-025-60435-8


