Science Highlights Archives
Anti-Inflammatory Antibodies Engineered to Treat Autoimmune Disease
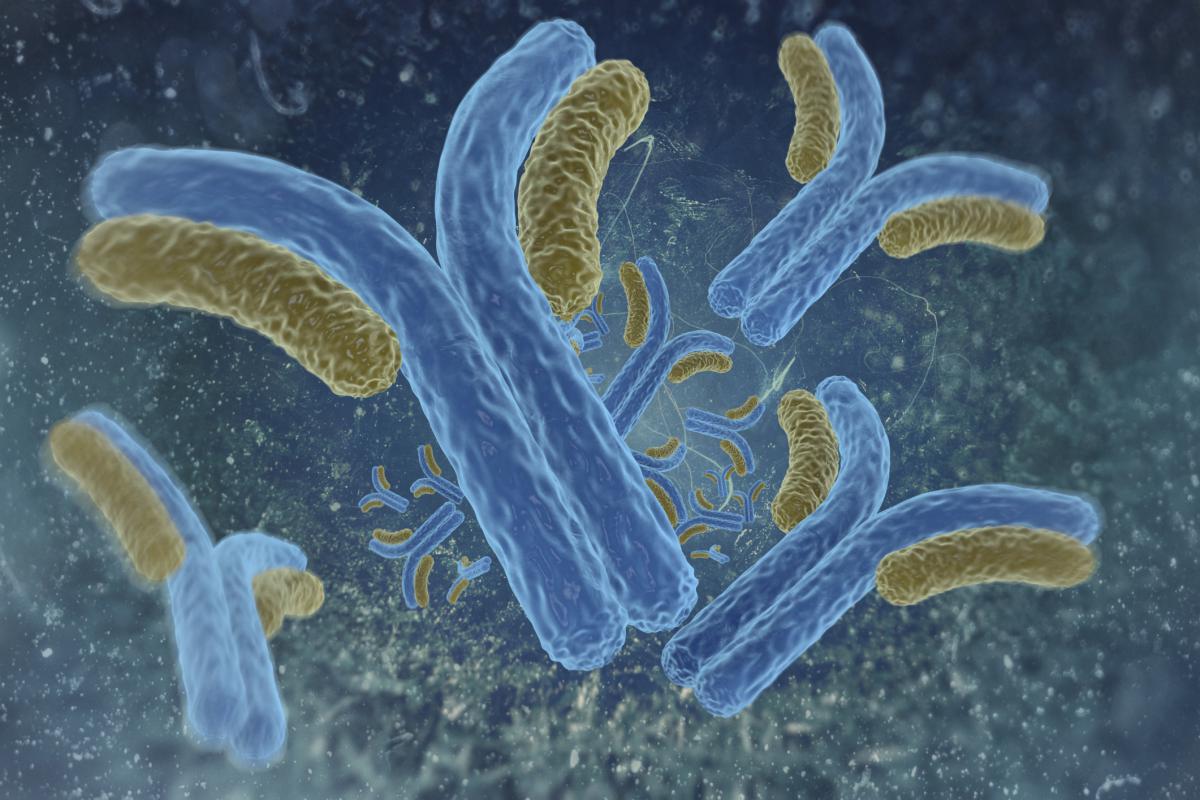 Robert Anthony’s (2014 New Innovator) laboratory engineered glycosylation enzymes that can transform rogue antibodies causing autoimmune disease into beneficial anti-inflammatory antibodies by modifying sugars attached to them. Treating an autoimmune disease often relies on reducing the inflammation caused from the immune system’s antibodies attacking the body’s own healthy cells, and, paradoxically, high doses of antibodies collected from healthy donors and administered intravenously is often used for treatment (called high-dose intravenous immunoglobin). But treatment requires large amounts of the antibodies, which are in short supply and expensive, and takes a long time to administer. Researchers observed that the anti-inflammatory effect of high-dose intravenous immunoglobin is attributed to a small portion of antibodies with sialic acid (a complex carbohydrate called a glycan) attached to the antibody. Sialic acid is usually attached to antibody proteins while they are being assembled in cells, but Anthony wondered if enzymes capable of adding chemical groups like glycans could attach sialic acid to antibodies outside the initial assembly process. Because sialic acid requires another glycan called galactose to attach to antibodies, Anthony’s team engineered two glycosylation enzymes to activate the attachment of sialic acid to other antibodies– one fused with an enzyme for sialic acid and one for galactose. When the enzyme antibodies were administered together in a mouse model of rheumatoid arthritis, the anti-inflammatory effects were similar to treatments with high-dose intravenous immunoglobin, and there was a reduction in kidney damage in a mouse model of lupus. The treatment appears to be safe, is specific to the site of inflammation, and does not affect other antibodies or glycans in circulation. The enzyme antibodies are effective at a 400-fold lower dose than high-dose intravenous immunoglobin and greatly reduces treatment time. Anthony’s team is now exploring treatment dosage, frequency, and administration method of the antibody enzymes as a therapy for autoimmune disease.
Robert Anthony’s (2014 New Innovator) laboratory engineered glycosylation enzymes that can transform rogue antibodies causing autoimmune disease into beneficial anti-inflammatory antibodies by modifying sugars attached to them. Treating an autoimmune disease often relies on reducing the inflammation caused from the immune system’s antibodies attacking the body’s own healthy cells, and, paradoxically, high doses of antibodies collected from healthy donors and administered intravenously is often used for treatment (called high-dose intravenous immunoglobin). But treatment requires large amounts of the antibodies, which are in short supply and expensive, and takes a long time to administer. Researchers observed that the anti-inflammatory effect of high-dose intravenous immunoglobin is attributed to a small portion of antibodies with sialic acid (a complex carbohydrate called a glycan) attached to the antibody. Sialic acid is usually attached to antibody proteins while they are being assembled in cells, but Anthony wondered if enzymes capable of adding chemical groups like glycans could attach sialic acid to antibodies outside the initial assembly process. Because sialic acid requires another glycan called galactose to attach to antibodies, Anthony’s team engineered two glycosylation enzymes to activate the attachment of sialic acid to other antibodies– one fused with an enzyme for sialic acid and one for galactose. When the enzyme antibodies were administered together in a mouse model of rheumatoid arthritis, the anti-inflammatory effects were similar to treatments with high-dose intravenous immunoglobin, and there was a reduction in kidney damage in a mouse model of lupus. The treatment appears to be safe, is specific to the site of inflammation, and does not affect other antibodies or glycans in circulation. The enzyme antibodies are effective at a 400-fold lower dose than high-dose intravenous immunoglobin and greatly reduces treatment time. Anthony’s team is now exploring treatment dosage, frequency, and administration method of the antibody enzymes as a therapy for autoimmune disease.
Reference: Engineered Sialylation of Pathogenic Antibodies In Vivo Attenuates Autoimmune Disease. Pagan JD, Kitaoka M, Anthony RM. Cell. 2017 Dec 11;pii: S0092-8674(17)31431-9.
In the News:
From Toy to Science Tool
 Centrifuges, a device that spins tubes of liquid at high speeds, are vital pieces of laboratory equipment but are often too expensive or impractical in many rural clinics around the world. Prakash Manu, a 2015 New Innovator, realized the need for an inexpensive centrifuge that didn’t require electricity and looked for a solution. He tested kitchen tools and children’s toys as centrifuge replacements and eventually settled on the whirligig, a child’s toy featuring a disc that is spun on string. Using the whirligig, Prakash created a hand-powered centrifuge capable of spinning at speeds of 125,000 rpm, fast enough to separate blood into its individual components in 1.5 minutes. Using only paper, twine, and plastic, the centrifuge costs 20 cents, is easily assembled, and is durable. Field trials on the “paperfuge” are currently underway and could be used in the diagnosis of diseases such as malaria and African sleeping sickness. The “paperfuge” is another step in Prakash’s efforts to make scientific tools more accessible to people around the world.
Centrifuges, a device that spins tubes of liquid at high speeds, are vital pieces of laboratory equipment but are often too expensive or impractical in many rural clinics around the world. Prakash Manu, a 2015 New Innovator, realized the need for an inexpensive centrifuge that didn’t require electricity and looked for a solution. He tested kitchen tools and children’s toys as centrifuge replacements and eventually settled on the whirligig, a child’s toy featuring a disc that is spun on string. Using the whirligig, Prakash created a hand-powered centrifuge capable of spinning at speeds of 125,000 rpm, fast enough to separate blood into its individual components in 1.5 minutes. Using only paper, twine, and plastic, the centrifuge costs 20 cents, is easily assembled, and is durable. Field trials on the “paperfuge” are currently underway and could be used in the diagnosis of diseases such as malaria and African sleeping sickness. The “paperfuge” is another step in Prakash’s efforts to make scientific tools more accessible to people around the world.
- Hand-Powered Ultralow-Cost Paper Centrifuge. Saad Bhamla M, et al. Nature Biomedical Engineering. 2017 Jan 10;1(0009):1-7.
- In the News: 20-Cent, Whirligig-Inspired Paperfuge Could Help Diagnose Diseases
- In the News: Children's Whirligig Toy Inspires a Low-Cost Laboratory Test
- In the News: An Ancient Toy Could Improve Health Care in the Developing World
Vaccine Candidate for Group A Streptococcus
 Rommie Amaro, a 2010 New Innovator, discovered a protein that may lead to a vaccine for the bacteria group A Streptococcus, a leading cause of worldwide morbidity and mortality and responsible for infections and diseases such as pharyngitis, rheumatic heart disease, and necrotizing fasciitis (“flesh-eating” disease). No vaccines currently exist against the bacteria, and efforts are hampered by the bacteria’s high variation of surface proteins, which activate the body’s immune response and are typically targeted for vaccine development. With over 200 distinct variations in surface proteins in group A Streptococcus, developing a vaccine able to recognize them all and confer universal immunity is unlikely. However, the discovery of a protein that interacts with the surface proteins offers hope. Researchers identified a protein called human C4b-binding protein (C4BP) that is brought to the surface of the bacteria to help block the host’s immune response. When tested against surface proteins, C4BP could interact with approximately 90% of group A Streptococcus strains. Analysis of surface protein structures found hidden patterns that allow C4BP to bind to them. The interaction between C4BP and surface proteins can be exploited for developing treatments, and the common sequence in the surface proteins is a valuable target for vaccine development against the bacteria.
Rommie Amaro, a 2010 New Innovator, discovered a protein that may lead to a vaccine for the bacteria group A Streptococcus, a leading cause of worldwide morbidity and mortality and responsible for infections and diseases such as pharyngitis, rheumatic heart disease, and necrotizing fasciitis (“flesh-eating” disease). No vaccines currently exist against the bacteria, and efforts are hampered by the bacteria’s high variation of surface proteins, which activate the body’s immune response and are typically targeted for vaccine development. With over 200 distinct variations in surface proteins in group A Streptococcus, developing a vaccine able to recognize them all and confer universal immunity is unlikely. However, the discovery of a protein that interacts with the surface proteins offers hope. Researchers identified a protein called human C4b-binding protein (C4BP) that is brought to the surface of the bacteria to help block the host’s immune response. When tested against surface proteins, C4BP could interact with approximately 90% of group A Streptococcus strains. Analysis of surface protein structures found hidden patterns that allow C4BP to bind to them. The interaction between C4BP and surface proteins can be exploited for developing treatments, and the common sequence in the surface proteins is a valuable target for vaccine development against the bacteria.
Conserved Patterns Hidden within Group A Streptococcus M Protein Hypervariability Recognize Human C4b-Binding Protein. Buffalo CZ et al. Nat Microbiol. 2016 Sept 5; 1(11):16155.
Visualizing RNA using Expansion Microscopy
 RNA molecules act as messengers, carrying copies of genetic instructions from the DNA throughout the cell and are crucial for protein synthesis and proper cell functioning. Arjun Raj (a 2011 New Innovator) and Edward Boyden (a 2007 New Innovator, 2012 and 2013 Transformative Researcher, and 2013 Pioneer) describe a new technique that can image RNA in cells and tissue by using “expansion microscopy,” a method where samples are physically enlarged with an expandable polymer, making high resolution imaging possible with common laboratory microscopes. Knowing the distribution of RNA inside cells will enable scientists to better understand how cells control gene expression and could aid study of diseases caused by the improper movement of RNA.
RNA molecules act as messengers, carrying copies of genetic instructions from the DNA throughout the cell and are crucial for protein synthesis and proper cell functioning. Arjun Raj (a 2011 New Innovator) and Edward Boyden (a 2007 New Innovator, 2012 and 2013 Transformative Researcher, and 2013 Pioneer) describe a new technique that can image RNA in cells and tissue by using “expansion microscopy,” a method where samples are physically enlarged with an expandable polymer, making high resolution imaging possible with common laboratory microscopes. Knowing the distribution of RNA inside cells will enable scientists to better understand how cells control gene expression and could aid study of diseases caused by the improper movement of RNA.
- Nanoscale Imaging of RNA with Expansion Microscopy. Chen F et al. Nat Methods. 2016 Aug 13;13(8):679-84.
- In the News: Nanoscale Microscopy Technique Allows Scientists to Pinpoint RNA Molecules in the Brain
An Alternative Way to Smell
 Sandeep Datta, a 2010 New Innovator, discovered alternative odor receptors in mice that upends previous conceptions about smell and reveals a much more complex system than previously believed. A single receptor family was thought to detect a single odor, but Datta found a group of neurons surrounding the olfactory bulb have an alternative mechanism for odor detection. These “necklace” neurons respond to odors with instinctive responses, such as pheromones and seeds and nuts, and demonstrate smell can be mediated by multiple types of receptors.
Sandeep Datta, a 2010 New Innovator, discovered alternative odor receptors in mice that upends previous conceptions about smell and reveals a much more complex system than previously believed. A single receptor family was thought to detect a single odor, but Datta found a group of neurons surrounding the olfactory bulb have an alternative mechanism for odor detection. These “necklace” neurons respond to odors with instinctive responses, such as pheromones and seeds and nuts, and demonstrate smell can be mediated by multiple types of receptors.
- A Family of non-GPCR Chemosensors Defines an Alternative Logic for Mammalian Olfaction. Greer PL et al. Cell. 2016 Jun 16;165(7):1734-48.
- In the News: Alternative Odor Receptors Discovered in Mice
- In the News: Odor Alternative
Defects in Neurons Reveal Clues to Autism
 Alysson Muotri, a 2009 New Innovator, reprogrammed stem cells to create neurons from autistic individuals who exhibited faster growing brains early on in life. The stem cell-derived neurons made fewer connections compared to cells from healthy individuals. Communication between the neurons was restored by adding IGF-1, a drug currently being tested for clinical treatment of autism. The use of stem cell reprogramming opens the way to modeling early stages of disease and to evaluate potential therapeutic treatments.
Alysson Muotri, a 2009 New Innovator, reprogrammed stem cells to create neurons from autistic individuals who exhibited faster growing brains early on in life. The stem cell-derived neurons made fewer connections compared to cells from healthy individuals. Communication between the neurons was restored by adding IGF-1, a drug currently being tested for clinical treatment of autism. The use of stem cell reprogramming opens the way to modeling early stages of disease and to evaluate potential therapeutic treatments.
Altered Proliferation and Networks in Neural Cells Derived from Idiopathic Autistic Individualss. Marchetto MC et al. Mol Psychiatry. 2016 Jul 5. doi: 10.1038/mp.2016.95.
When Molecular Machinery Collide
 Jue Wang, a 2008 New Innovator, discovered what happens when a cell makes copies of its DNA at the same time it is translating its DNA into mRNA and the molecular machinery collide. A specific gene in bacteria was engineered so either the process of replication and transcription proceeded in the same DNA strand direction or so the processes would collide head-on. Wang found that when replication and transcription collided head-on, the mutation rate was higher than when their paths followed the same direction. Most of the mutations from the collision were either insertions/deletions or substitutions in the promoter region of the gene, which may drastically affect protein production and an organism’s health.
Jue Wang, a 2008 New Innovator, discovered what happens when a cell makes copies of its DNA at the same time it is translating its DNA into mRNA and the molecular machinery collide. A specific gene in bacteria was engineered so either the process of replication and transcription proceeded in the same DNA strand direction or so the processes would collide head-on. Wang found that when replication and transcription collided head-on, the mutation rate was higher than when their paths followed the same direction. Most of the mutations from the collision were either insertions/deletions or substitutions in the promoter region of the gene, which may drastically affect protein production and an organism’s health.
- The Nature of Mutations Induced by Replication–Transcription Collisions. Sankar TS, Wastuwidyaningtyas BD, Dong Y, Lewis SA, Wang JD. Nature. 2016 Jul 7;535(7610):178-81.
- In the News: Collisions During DNA Replication and Transcription Contribute to Mutagenesis
Building Cellular Circuits
 Timothy Lu, a 2011 New Innovator, designed cells to remember and respond to a series of events, creating biological “state machines.” When cells are activated by a specific input, enzymes called recombinases either delete or invert a particular stretch of DNA. The affected DNA contains further sites for other recombinases to act on depending on an additional input, and so on. The cell’s history can then be read by sequencing its DNA. Lu built circuits for 3 inputs with 16 possible states. The ability to respond and record combinations of biological events and their order provides researchers with a tool to study temporal organization in cell regulation and disease progression.
Timothy Lu, a 2011 New Innovator, designed cells to remember and respond to a series of events, creating biological “state machines.” When cells are activated by a specific input, enzymes called recombinases either delete or invert a particular stretch of DNA. The affected DNA contains further sites for other recombinases to act on depending on an additional input, and so on. The cell’s history can then be read by sequencing its DNA. Lu built circuits for 3 inputs with 16 possible states. The ability to respond and record combinations of biological events and their order provides researchers with a tool to study temporal organization in cell regulation and disease progression.
- Synthetic Recombinase-Based State Machines in Living Cells. Roquet N, Soleimany AP, Ferris AC, Aaronson S, Lu TK. Science. 2016 Jul 22;353(6297):aad8559. doi: 10.1126/science.aad8559.
Creating Memory Loss
 Joanna Jankowsky, a 2007 New Innovator, showed that spatial memory decays when the entorhinal cortex in the brain malfunctions. The entorhinal cortex funnels sensory information to the hippocampus where the information is bound together to form a memory and function in spatial navigation. The entorhinal cortex in a mouse model was genetically engineered with cell receptors that could be silenced with the drug ivermectin. When the entorhinal cortex was turned off, distinct brain patterns coding for specific spatial locations were altered, and the mice couldn’t successfully complete mazes they had previously learned. The findings provides more clarity on how the hippocampus is required to learn a new environment and recall it, and provides a model for studying conditions like Alzheimer’s.
Joanna Jankowsky, a 2007 New Innovator, showed that spatial memory decays when the entorhinal cortex in the brain malfunctions. The entorhinal cortex funnels sensory information to the hippocampus where the information is bound together to form a memory and function in spatial navigation. The entorhinal cortex in a mouse model was genetically engineered with cell receptors that could be silenced with the drug ivermectin. When the entorhinal cortex was turned off, distinct brain patterns coding for specific spatial locations were altered, and the mice couldn’t successfully complete mazes they had previously learned. The findings provides more clarity on how the hippocampus is required to learn a new environment and recall it, and provides a model for studying conditions like Alzheimer’s.
- Impaired Recall of Positional Memory following Chemogenetic Disruption of Place Field Stability. Zhao R, et al. Cell Rep. 2016 Jul 19;16(3):793-804.
Ultra-Resolution Microscopy
 Peng Yin, a 2010 New Innovator and 2013 Transformative Research awardee, enhanced DNA nanotechnology-powered super-resolution microscopy to visualize distinct molecules only five nanometers apart. The ultra-high resolution is achieved through a novel method that compensates for movements of the microscope stage and by further harnessing the power of DNA-PAINT, which is based on the transient binding of short strands of complementary DNA, one which is tagged with a fluorescent dye and the other is attached to the molecule of interest. The method opens the way for researchers to study individual molecular features in molecular complexes that may ultimately lead to a greater understanding of their functions.
Peng Yin, a 2010 New Innovator and 2013 Transformative Research awardee, enhanced DNA nanotechnology-powered super-resolution microscopy to visualize distinct molecules only five nanometers apart. The ultra-high resolution is achieved through a novel method that compensates for movements of the microscope stage and by further harnessing the power of DNA-PAINT, which is based on the transient binding of short strands of complementary DNA, one which is tagged with a fluorescent dye and the other is attached to the molecule of interest. The method opens the way for researchers to study individual molecular features in molecular complexes that may ultimately lead to a greater understanding of their functions.
- Optical Imaging of Individual Biomolecules in Densely Packed Clusters. Dai M, Jungmann R, Yin P. Nat Nanotechnol. 2016 Jul 4. doi: 10.1038/nnano.2016.95.
- In the News: From Super to Ultra-Resolution Microscopy
Finding the Needle in a Haystack: New Tool to Find Significant Results from Genome-Wide Association Data
 Genome-wide association studies (GWAS) have the ability to reveal thousands of genetic variants associated with a particular disease or trait (cancer, height, etc.). While GWAS studies have become a powerful new tool for researchers, they also reveal thousands of potential variants of which many are not directly related to a condition but merely associated. Finding the genetic changes that drive a disease or trait can be difficult, especially when the variants are in non-coding regions of the genome. While non-coding regions of the genome do not directly express genes, they often regulate the expression of coding areas. Dr. Sabeti, New Innovator Awardee, and colleagues have devised a new tool that can directly identify which variants are affecting expression on a very large scale. The group was able to take an existing technology (published as a companion paper in the same issue of Cell) and scale it to examine more variants while also reducing noise and increasing the sensitivity of the test. This new resource should prove to be highly effective to many researchers who already have access to GWAS data but are having difficulty interpreting their results.
Genome-wide association studies (GWAS) have the ability to reveal thousands of genetic variants associated with a particular disease or trait (cancer, height, etc.). While GWAS studies have become a powerful new tool for researchers, they also reveal thousands of potential variants of which many are not directly related to a condition but merely associated. Finding the genetic changes that drive a disease or trait can be difficult, especially when the variants are in non-coding regions of the genome. While non-coding regions of the genome do not directly express genes, they often regulate the expression of coding areas. Dr. Sabeti, New Innovator Awardee, and colleagues have devised a new tool that can directly identify which variants are affecting expression on a very large scale. The group was able to take an existing technology (published as a companion paper in the same issue of Cell) and scale it to examine more variants while also reducing noise and increasing the sensitivity of the test. This new resource should prove to be highly effective to many researchers who already have access to GWAS data but are having difficulty interpreting their results.
References
Broad Institute News: A massive approach to finding what's "real" in genome-wide association data
Direct Identification of Hundreds of Expression-Modulating Variants using a Multiplexed Reporter Assay. Tewhey R, Kotliar D, Park DS, Liu B, Winnicki S, Reilly SK, Andersen KG, Mikkelsen TS, Lander ES, Schaffner SF, and Sabeti PC. Cell. 2016 Jun 2; 65(6):1519-29.
Histone Mutation Linked to Pediatric Cancers
 Benjamin Garcia, 2010 New Innovator, and Ping Chi, a 2012 New Innovator, found a link between a single mutation in a gene coding for a histone and pediatric cancers. The mutation blocks specialization in the type of stem cell that can form cartilage, bone, and fat. Mice with the mutation developed undifferentiated pediatric sarcoma. They also found 20% of human tissue from undifferentiated sarcomas had the mutation. Their work is the first to demonstrate a histone gene mutation can cause cancer by itself and provides a potential drug target in the fight against cancer.
Benjamin Garcia, 2010 New Innovator, and Ping Chi, a 2012 New Innovator, found a link between a single mutation in a gene coding for a histone and pediatric cancers. The mutation blocks specialization in the type of stem cell that can form cartilage, bone, and fat. Mice with the mutation developed undifferentiated pediatric sarcoma. They also found 20% of human tissue from undifferentiated sarcomas had the mutation. Their work is the first to demonstrate a histone gene mutation can cause cancer by itself and provides a potential drug target in the fight against cancer.
- Histone H3K36 Mutations Promote Sarcomagenesis through Altered Histone Methylation Landscape. Lu C et al. Science. 2016 May 13;352(6287):844-9.
Genome Architecture Regulated through Topologically Associated Domains
 Steven Kosak, a 2011 New Innovator, tested the relationship between the linear and 3D organization of gene regulation during muscle formation and found them to be linked through topologically associated domain (TAD) architecture. TADs are a unifying structural model for chromatin organization, and Kosak found they influence and inform cell-specific genome organization. Changes in organization influence transcription and require cell division to be established.
Steven Kosak, a 2011 New Innovator, tested the relationship between the linear and 3D organization of gene regulation during muscle formation and found them to be linked through topologically associated domain (TAD) architecture. TADs are a unifying structural model for chromatin organization, and Kosak found they influence and inform cell-specific genome organization. Changes in organization influence transcription and require cell division to be established.
Topologically Associated Domains Enriched for Lineage-Specific Genes Reveal Expression-Dependent Nuclear Topologies during Myogenesis. Neems DS, Garza-Gongoar AG, Smith ED, Kosak ST. Proc Natl Acad Sci U S A. 2016 Mar 22;113(12):E1691-700.
The Importance of DNA Folding in Stem Cells
 Jennifer Phillips-Cremins, a 2015 New Innovator, published a paper exploring why lab-made stem cells may fail to correctly turn back into adult cells: The genomes in the cells retain folding patterns that partially resemble the adult cells’ folded DNA from which they were made. Her lab mapped the folded pattern of genomes by chemically fixing the folded genome so sections of linear genetic sequence are fixed near spatially adjacent (but not sequentially adjacent) pieces of DNA. The spatially adjacent pieces end up in the same string of hybrid DNA and are detected together when the DNA is sequenced. These hybrid pieces can be used to infer which DNA segments are adjacent to each other in the genome’s folded state, elucidating the 3D genome. Phillips-Cremins also found they could make more accurate folding states in stem cells by changing the medium they are grown in. Her research opens new possibilities in stem cell engineering for experiments and treatments.
Jennifer Phillips-Cremins, a 2015 New Innovator, published a paper exploring why lab-made stem cells may fail to correctly turn back into adult cells: The genomes in the cells retain folding patterns that partially resemble the adult cells’ folded DNA from which they were made. Her lab mapped the folded pattern of genomes by chemically fixing the folded genome so sections of linear genetic sequence are fixed near spatially adjacent (but not sequentially adjacent) pieces of DNA. The spatially adjacent pieces end up in the same string of hybrid DNA and are detected together when the DNA is sequenced. These hybrid pieces can be used to infer which DNA segments are adjacent to each other in the genome’s folded state, elucidating the 3D genome. Phillips-Cremins also found they could make more accurate folding states in stem cells by changing the medium they are grown in. Her research opens new possibilities in stem cell engineering for experiments and treatments.
- Local Genome Topology Can Exhibit an Incompletely Rewired 3D-Folding State during Somatic Cell Reprogramming. Beagan JA et al. Cell Stem Cell. 2016 May 5;18(5):611-24.
Untangling Neurons
 Viviana Gradinaru, a 2013 New Innovator, untangled highly intermingled neuronal circuits in a region of the brain implicated in motor impairment (found in Parkinson’s disease patients) and addiction. Neurons were mapped using a technique developed by the Grandinaru lab called Passive CLARITY Technique, which dissolves lipids in tissue and makes the brain optically transparent. By marking the neuron with fluorescent proteins, each neuron path could be followed through the brain. The affected behavior was determined through manipulation of the neural activity using optogenetics, which can turn a neuron on or off using light. The techniques enable brain-wide functional and anatomical mapping of neuron complexes that can help inform future treatments for diseases like Parkinson’s.
Viviana Gradinaru, a 2013 New Innovator, untangled highly intermingled neuronal circuits in a region of the brain implicated in motor impairment (found in Parkinson’s disease patients) and addiction. Neurons were mapped using a technique developed by the Grandinaru lab called Passive CLARITY Technique, which dissolves lipids in tissue and makes the brain optically transparent. By marking the neuron with fluorescent proteins, each neuron path could be followed through the brain. The affected behavior was determined through manipulation of the neural activity using optogenetics, which can turn a neuron on or off using light. The techniques enable brain-wide functional and anatomical mapping of neuron complexes that can help inform future treatments for diseases like Parkinson’s.
- Cholinergic Mesopontine Signals Govern Locomotion and Reward through Dissociable Midbrain Pathways. Xiao C et al. Neuron. 2016 Apr 20;90(2):333-47.
Counting Molecules with qPAINT
 Peng Yin (2010 New Innovator and 2013 Transformative Researcher) and Avital Rodal (2012 New Innovator) published a paper in Nature Methods describing enhancements in optical super-resolution microscopy for counting molecules in complexes. Quantitative points accumulation in nanoscale topography (qPAINT) improves DNA-PAINT by working independently from dye photophysics for robust counting with high precision and accuracy over a wide dynamic range. The method is immune to photobleaching and is capable of multiplexing for a large number of targets.
Peng Yin (2010 New Innovator and 2013 Transformative Researcher) and Avital Rodal (2012 New Innovator) published a paper in Nature Methods describing enhancements in optical super-resolution microscopy for counting molecules in complexes. Quantitative points accumulation in nanoscale topography (qPAINT) improves DNA-PAINT by working independently from dye photophysics for robust counting with high precision and accuracy over a wide dynamic range. The method is immune to photobleaching and is capable of multiplexing for a large number of targets.
- Quantitative Super-Resolution Imaging with qPAINT. Jungmann R et al. Nat Methods. 2016 May;13(5):439-42.
Making Liposomes
 William Shih (2008 New Innovator) and Chenxiang Lin (2014 New Innovator) developed a technique to create tiny, membrane-bound vesicles called liposomes. Using “DNA-origami,” liposomes were fabricated by folding single-stranded DNA into a customized shape with the help of short staple strands of DNA. Lipid molecules were then placed inside the structure to help guide the formation of liposomes from 29 to 94 nanometers in size. The ability to fabricate specific sizes of liposomes enables scientists to study how cells and subcellular compartments interact and may eventually enable efficient drug delivery to target cells.
William Shih (2008 New Innovator) and Chenxiang Lin (2014 New Innovator) developed a technique to create tiny, membrane-bound vesicles called liposomes. Using “DNA-origami,” liposomes were fabricated by folding single-stranded DNA into a customized shape with the help of short staple strands of DNA. Lipid molecules were then placed inside the structure to help guide the formation of liposomes from 29 to 94 nanometers in size. The ability to fabricate specific sizes of liposomes enables scientists to study how cells and subcellular compartments interact and may eventually enable efficient drug delivery to target cells.
- Self-Assembly of Size-Controlled Liposomes on DNA Nanotemplates. Yang Y et al. Nat Chem. 2016 May;8(5):476-83.
Shortening Time between Vaccine Boosters
 Vaiva Vezys, a 2009 New Innovator, found shortened time between vaccination boosters still enables functional and protective antigen-specific CD8 T cells. Current heterologous prime-boost-boost vaccination strategies generate large numbers of long-lasting memory CD8 T cells but require at least 28 days to 6 months between boosters and can leave the host vulnerable for an extensive period of time during the process. By shortening the length of time between boosts to 2 weeks, Vezys showed protection comparable to the long-term boosting intervals was still achieved. The shortened boosts did not form stable long-term memory, but the shorter boosting time may help increase vaccination compliance and could be useful in situations where protection needs to be achieved rapidly, such as in disease outbreaks.
Vaiva Vezys, a 2009 New Innovator, found shortened time between vaccination boosters still enables functional and protective antigen-specific CD8 T cells. Current heterologous prime-boost-boost vaccination strategies generate large numbers of long-lasting memory CD8 T cells but require at least 28 days to 6 months between boosters and can leave the host vulnerable for an extensive period of time during the process. By shortening the length of time between boosts to 2 weeks, Vezys showed protection comparable to the long-term boosting intervals was still achieved. The shortened boosts did not form stable long-term memory, but the shorter boosting time may help increase vaccination compliance and could be useful in situations where protection needs to be achieved rapidly, such as in disease outbreaks.
- Shortened Intervals during Heterologous Boosting Preserve Memory CD8 T Cell Function but Compromise Longevity. Thompson EA, Beura LK, Nelson CE, Anderson KG, Vezys V. J Immunol. 2016 Apr 1;196(7):3054-63.
Tuberculosis and Smoking
 Ira Hall, a 2009 New Innovator, discovered a possible mechanism explaining why smokers are more susceptible to tuberculosis. Using a zebrafish tuberculosis model with Mycobacterium marinum, Hall examined macrophage response to mycobacterial infection. During mycobacterial infection, macrophages with lysosomal storage cannot migrate to or engulf infected apoptotic macrophages, leading to secondary necrosis and increased mycobacterial growth. This phenotype is recapitulated in human smokers where alveolar macrophages accumulate tobacco smoke particulates in lysosomes, which impedes migration to and engulfment of Mycobacterium tuberculosis. The incapacitation of macrophages severely hampers the ability to fight the mycobacterium, and may explain why smokers are more susceptible to tuberculosis infection.
Ira Hall, a 2009 New Innovator, discovered a possible mechanism explaining why smokers are more susceptible to tuberculosis. Using a zebrafish tuberculosis model with Mycobacterium marinum, Hall examined macrophage response to mycobacterial infection. During mycobacterial infection, macrophages with lysosomal storage cannot migrate to or engulf infected apoptotic macrophages, leading to secondary necrosis and increased mycobacterial growth. This phenotype is recapitulated in human smokers where alveolar macrophages accumulate tobacco smoke particulates in lysosomes, which impedes migration to and engulfment of Mycobacterium tuberculosis. The incapacitation of macrophages severely hampers the ability to fight the mycobacterium, and may explain why smokers are more susceptible to tuberculosis infection.
- Lysosomal Disorders Drive Susceptibility to Tuberculosis by Compromising Macrophage Migration. Berg RD et al. Cell. 2016 Mar 24;165(1):139-52.
Mutations in Neurons
 David Tobin, a 2011 New Innovator, documented somatic mutations in neurons through whole-genome sequencing using a nuclear transfer method to clonally amplify the genomes of neurons from adult mice. Neurons are of particular interest because they maintain their identity without cell division or replacement during the lifetime of the organism and face unique genomic threats due to their high metabolic rate and use of epigenetic chromatin remodeling and DNA breaks to alter gene expression. Tobin found individual neurons harbor ~100 unique mutations from all classes except recurrent rearrangements. Most neurons contain at least one gene-disrupting mutation and rare mobile element insertions. The frequency and gene bias of neuronal mutations differ from other lineages, possibly due to novel mechanisms governing postmitotic mutation. Tobin was further able to clone fully fertile mice from several neurons, showing mutated adult neuronal genomes are compatible with reprogramming.
David Tobin, a 2011 New Innovator, documented somatic mutations in neurons through whole-genome sequencing using a nuclear transfer method to clonally amplify the genomes of neurons from adult mice. Neurons are of particular interest because they maintain their identity without cell division or replacement during the lifetime of the organism and face unique genomic threats due to their high metabolic rate and use of epigenetic chromatin remodeling and DNA breaks to alter gene expression. Tobin found individual neurons harbor ~100 unique mutations from all classes except recurrent rearrangements. Most neurons contain at least one gene-disrupting mutation and rare mobile element insertions. The frequency and gene bias of neuronal mutations differ from other lineages, possibly due to novel mechanisms governing postmitotic mutation. Tobin was further able to clone fully fertile mice from several neurons, showing mutated adult neuronal genomes are compatible with reprogramming.
- The Complete Genome Sequences, Unique Mutational Spectra, and Developmental Potency of Adult Neurons Revealed by Cloning. Hazen JL et al. Neuron. 2016 Mar 16;89(6):1223-36.
Fruit Flies Adjust Courtship Song Based on Distance
 Mala Murthy, a 2014 New Innovator, discovered male fruit flies are able to adjust the intensity of their courtship song to compensate for their distance from the female (such control was previously identified in only humans and songbirds). Murthy analyzed each stage of the neuronal pathway underlying the male’s ability to adjust his courtship song, from visual cues to estimate distance to signals sent through the nervous system to change muscle activity to create a louder or softer song. Studying the process and mechanisms behind this behavior in the fruit fly can help illuminate the building blocks for social interactions across the animal kingdom.
Mala Murthy, a 2014 New Innovator, discovered male fruit flies are able to adjust the intensity of their courtship song to compensate for their distance from the female (such control was previously identified in only humans and songbirds). Murthy analyzed each stage of the neuronal pathway underlying the male’s ability to adjust his courtship song, from visual cues to estimate distance to signals sent through the nervous system to change muscle activity to create a louder or softer song. Studying the process and mechanisms behind this behavior in the fruit fly can help illuminate the building blocks for social interactions across the animal kingdom.
- Sensorimotor Transformations Underlying Variability in Song Intensity during Drosophila Courtship. Coen P, Xie M, Cemens J, and Murthy M. Neuron. 2016 Feb 3;89(3):629-44.
- In the News: Fruit Flies Adjust Their Courtship Song Based on Distance
Gene Therapy Across the Blood-Brain Barrier
 Viviana Gradinaru, a 2013 New Innovator, modified a virus so it is capable of crossing the blood-brain barrier to deliver genes to nervous system cells. Gradinaru made millions of modifications to the protein shell of a small, harmless virus called an adeno-associated virus and determined which variants most effectively delivered genes to astrocytes in the brain by searching for the delivered gene load. The process was repeated until the most effective viruses were found. The virus could help researchers map the brain and be used to deliver gene therapeutics for diseases such as Alzheimer's and Huntington's.
Viviana Gradinaru, a 2013 New Innovator, modified a virus so it is capable of crossing the blood-brain barrier to deliver genes to nervous system cells. Gradinaru made millions of modifications to the protein shell of a small, harmless virus called an adeno-associated virus and determined which variants most effectively delivered genes to astrocytes in the brain by searching for the delivered gene load. The process was repeated until the most effective viruses were found. The virus could help researchers map the brain and be used to deliver gene therapeutics for diseases such as Alzheimer's and Huntington's.
- Cre-Dependent Selection Yields AAV Variants for Widespread Gene Transfer to the Adult Brain. Deverman BE et al. Nat Biotechnol. 2016 Feb 1. doi: 10.1038/nbt.3440.
- In the News: Delivering Genes Across the Blood-Brain Barrier
Infection Survival Influenced by Nutrient Intake
 Julie Canman, a 2011 New Innovator, published a paper identifying a novel link between nutrient intake and tolerance of infection to the bacterial pathogen Burkholderia cepacia (important in hospital-acquired infections) in Drosophila circadian period mutants. Tolerance to infection was stimulated with exposure to dietary glucose and amino acids. Canman investigated the role of the amino-acid-sensing pathway Target of Rapamycin (TOR) in immunity and found TORC1 inhibition decreased resistance while inhibition of TORC2 increased resistance and tolerance. The TOR pathway offers a potential therapeutic target for survival of infection.
Julie Canman, a 2011 New Innovator, published a paper identifying a novel link between nutrient intake and tolerance of infection to the bacterial pathogen Burkholderia cepacia (important in hospital-acquired infections) in Drosophila circadian period mutants. Tolerance to infection was stimulated with exposure to dietary glucose and amino acids. Canman investigated the role of the amino-acid-sensing pathway Target of Rapamycin (TOR) in immunity and found TORC1 inhibition decreased resistance while inhibition of TORC2 increased resistance and tolerance. The TOR pathway offers a potential therapeutic target for survival of infection.
- Period-Regulated Feeding Behavior and TOR Signaling Modulate Survival of Infection. Allen VW et al. Curr Biol. 2016 Jan 25;26(2):184-94.
Using Gene Editing to Treat Duchenne Muscular Dystrophy
 Amy Wagers (2008 New Innovator), George Church (2013 Transformative Researcher), and Feng Zhang (2012 Pioneer; 2010 & 2015 Transformative Researcher) published back-to-back Science papers on using the CRISPR-Cas9 system to edit the genome of a mouse model of Duchenne muscular dystrophy and improving muscle function. The researchers loaded a virus with the DNA-cutting tool CRISPR and infected the mice's muscle cells. CRISPR excised a defective stretch of DNA, shortening the dystrophin protein and making it functional. The functional protein led to muscle improvement and increased strength in the mice and brings hope for one day using gene editing to treat human's with Duchenne Muscular Dystrophy.
Amy Wagers (2008 New Innovator), George Church (2013 Transformative Researcher), and Feng Zhang (2012 Pioneer; 2010 & 2015 Transformative Researcher) published back-to-back Science papers on using the CRISPR-Cas9 system to edit the genome of a mouse model of Duchenne muscular dystrophy and improving muscle function. The researchers loaded a virus with the DNA-cutting tool CRISPR and infected the mice's muscle cells. CRISPR excised a defective stretch of DNA, shortening the dystrophin protein and making it functional. The functional protein led to muscle improvement and increased strength in the mice and brings hope for one day using gene editing to treat human's with Duchenne Muscular Dystrophy.
- In Vivo Genome Editing Improves Muscle Function in a Mouse Model of Duchenne Muscular Dystrophy. Nelson CE et al. Science. 2016 Jan 22;351(6271):403-7.
- In Vivo Gene Editing in Dystrophic Mouse Muscle and Muscle Stem Cells. Tabebordbar M et al. Science. 2016 Jan 22;351(6271):407-11.
- In the News: Gene Editing Offers Hope for Treating Duchenne Muscular Dystrophy, Studies Find
- In the News: CRISPR Helps Heal Mice with Muscular Dystrophy
Silencing Chromosome X Genes by Xist RNA
 Sundeep Kalantry, a 2011 New Innovator, published a paper showing X chromosome inactivation in females is not solely due to the activity of X-inactive specific transcript (Xist) RNA as previously believed. Using Xist RNA induced mice, Kalantry found Xist RNA can silence X-linked genes in females but not in males. The results suggest that both Xist induction and X-linked gene silencing are orchestrated by the handful of genes that do not undergo X inactivation in females.
Sundeep Kalantry, a 2011 New Innovator, published a paper showing X chromosome inactivation in females is not solely due to the activity of X-inactive specific transcript (Xist) RNA as previously believed. Using Xist RNA induced mice, Kalantry found Xist RNA can silence X-linked genes in females but not in males. The results suggest that both Xist induction and X-linked gene silencing are orchestrated by the handful of genes that do not undergo X inactivation in females.
- Sex-Specific Silencing of X-Linked Genes by Xist RNA. Gayen S, Maclary E, Hinten M, and Kalantry S. Proc Natl Acad Sci USA. 2016 Jan 19;113(3):E309-18.
Mapping Body Language
 Sandeep Datta, a 2010 New Innovator, developed new computational techniques that read complex patterns of behavior and body movements of mice and organizes them into units. Using the video-game device Microsoft Kinect, Datta was able to capture mouse movements and recreate them on screen in 3D. With this data, he realized changes in the mouse’s posture and movement could be organized into distinct units with identifiable patterns. The techniques propose a solution to the longstanding problem in neuroscience of how to objectively study complex three-dimensional patterns of animal behavior without relying on subjective human observers, and Datta hopes the method will lead to new insight into how the brain creates behavior and how that process is disrupted in disease. View the video abstract below for more information.
Sandeep Datta, a 2010 New Innovator, developed new computational techniques that read complex patterns of behavior and body movements of mice and organizes them into units. Using the video-game device Microsoft Kinect, Datta was able to capture mouse movements and recreate them on screen in 3D. With this data, he realized changes in the mouse’s posture and movement could be organized into distinct units with identifiable patterns. The techniques propose a solution to the longstanding problem in neuroscience of how to objectively study complex three-dimensional patterns of animal behavior without relying on subjective human observers, and Datta hopes the method will lead to new insight into how the brain creates behavior and how that process is disrupted in disease. View the video abstract below for more information.
- Mapping Sub-Second Structure in Mouse Behavior. Wiltschko AB et al. Neuron. 2015 Dec 16;88(6):1121-35.
- In the News: Understanding Body Language (with video abstract)
Identifying Small RNA Targets
 Joseph Wade, a 2010 New Innovator, published a paper identifying regulatory targets of the Escherichia coli small RNA RhyB using ribosome profiling (Ribo-seq). Small RNAs typically regulate expression of messenger RNAs through base-pairing interactions, but identifying small RNA targets is difficult due to the short regions of base-pairing compared to the relatively long small RNAs, which can also be influenced by secondary structures. With Ribo-seq, Wade was able to validate a majority of known RyhB targets and discover many novel ones. The technique is a powerful approach for identifying small RNA targets genome-wide and can distinguish small RNA regulation at the level of messenger RNA stability or translation.
Joseph Wade, a 2010 New Innovator, published a paper identifying regulatory targets of the Escherichia coli small RNA RhyB using ribosome profiling (Ribo-seq). Small RNAs typically regulate expression of messenger RNAs through base-pairing interactions, but identifying small RNA targets is difficult due to the short regions of base-pairing compared to the relatively long small RNAs, which can also be influenced by secondary structures. With Ribo-seq, Wade was able to validate a majority of known RyhB targets and discover many novel ones. The technique is a powerful approach for identifying small RNA targets genome-wide and can distinguish small RNA regulation at the level of messenger RNA stability or translation.
- Identification of Bacterial sRNA Regulatory Targets Using Ribosome Profiling. Wang J et al. Nucleic Acids Res. 2015 Dec 2;43(21):10308-20.
Extending Youth in Worms
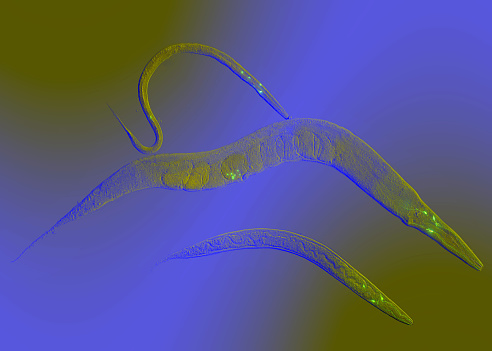 Michael Petrascheck, a 2011 New Innovator, used an antidepressant drug to prolong life in roundworms by 30% by extending young adulthood with no effect on later life stages. Petrascheck believes the drug help coordinate gene activity, which tends to become more disorganized during aging (termed "transcriptional drift"). While it is still far from being a cure for human aging, it raises the possibility of using drugs to reduce or delay the onset of age-related disease.
Michael Petrascheck, a 2011 New Innovator, used an antidepressant drug to prolong life in roundworms by 30% by extending young adulthood with no effect on later life stages. Petrascheck believes the drug help coordinate gene activity, which tends to become more disorganized during aging (termed "transcriptional drift"). While it is still far from being a cure for human aging, it raises the possibility of using drugs to reduce or delay the onset of age-related disease.
- Suppression of Transcriptional Drift Extends C. elegans Lifespan by Postponing the Onset of Mortality. Rangaraju S et al. ELife. 2015 Dec 1; 4.
Wake-Up Call to Drug Resistant Tuberculosis
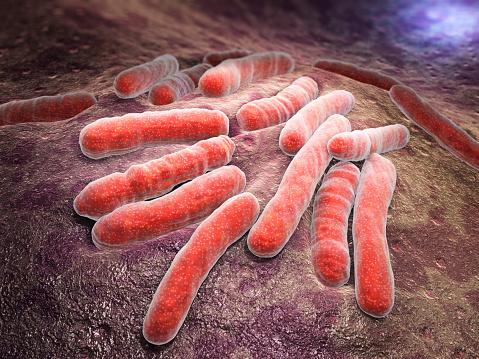 Sanjay Jain, a 2009 New Innovator and 2014 Transformative Researcher, reported successfully treating a child with extensively drug-resistant TB, or XDR-TB, in the United States. Treatment was difficult, even with vast resources available. Jain was able to successfully monitor and treat the young patient but warns the case is "a wake-up call to the realities of TB."
Sanjay Jain, a 2009 New Innovator and 2014 Transformative Researcher, reported successfully treating a child with extensively drug-resistant TB, or XDR-TB, in the United States. Treatment was difficult, even with vast resources available. Jain was able to successfully monitor and treat the young patient but warns the case is "a wake-up call to the realities of TB."
Extensively Drug-Resistant Tuberculosis in a Young Child after Travel to India. Salazar-Austin N et al. Lancet Infect Dis. 2015 Dec; 15(12):1485-91.
New Model Shows Maternal Immune Cells Protect Against Severe Cytomegalovirus Disease
 Sallie Permar, a 2012 New Innovator, developed a nonhuman primate model of congenital cytomegalovirus transmission (the leading infectious cause of childhood hearing loss and brain damage) and used the model to demonstrate the important role the maternal immune system plays in disease severity and fetal loss. Maternal CD4(+) T-cell immunity during infection control viremia and induce protective immune responses. The model opens the pathway for clinically-relevant congenital CMV vaccine development and research.
Sallie Permar, a 2012 New Innovator, developed a nonhuman primate model of congenital cytomegalovirus transmission (the leading infectious cause of childhood hearing loss and brain damage) and used the model to demonstrate the important role the maternal immune system plays in disease severity and fetal loss. Maternal CD4(+) T-cell immunity during infection control viremia and induce protective immune responses. The model opens the pathway for clinically-relevant congenital CMV vaccine development and research.
- Maternal CD4+ T Cells Protect Against Severe Congenital Cytomegalovirus Disease in a Novel Nonhuman Primate Model of Placental Cytomegalovirus Transmission. Bialas KM et al. Proc Natl Acad Sci USA. 2015 Nov 3; 112(44):13645-50.
Bacteriocins Alter Gut Bacteria Composition
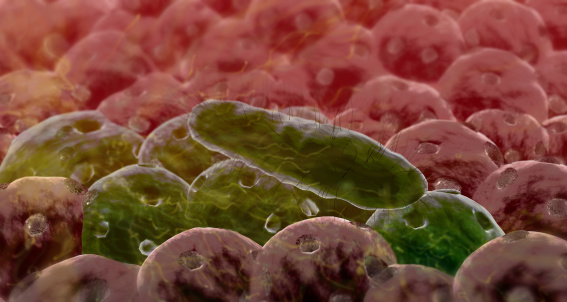 Christopher Kristich, a 2009 New Innovator, developed a model of colonization of the mouse gut with Enterococcus faecalis (a leading cause of hospital-acquired infections) to evaluate the role of plasmids encoding antibacterial proteins in enterococcal colonization. Kristich found that antibacterial proteins from commensal bacteria can influence niche competition in the gastrointestinal tract and that delivering antibacterial proteins by commensal bacteria occupying a precise intestinal niche may be an effective approach to eliminating colonization by multidrug-resistant bacteria without disrupting indigenous microbes.
Christopher Kristich, a 2009 New Innovator, developed a model of colonization of the mouse gut with Enterococcus faecalis (a leading cause of hospital-acquired infections) to evaluate the role of plasmids encoding antibacterial proteins in enterococcal colonization. Kristich found that antibacterial proteins from commensal bacteria can influence niche competition in the gastrointestinal tract and that delivering antibacterial proteins by commensal bacteria occupying a precise intestinal niche may be an effective approach to eliminating colonization by multidrug-resistant bacteria without disrupting indigenous microbes.
- Bacteriocin Production Augments Niche Competition by Enterococci in the Mammalian Gastrointestinal Tract. Kommineni S et al. Nature. Oct 29; 526(7575):719-22.
m6A mRNA Methylation Directs Translational Control of Heat Shock Response
 Shu-Bing Qian, a 2009 New Innovator, demonstrated the dynamic nature of N6-methyladenosine (m6A) modification to mRNAs, the most abundant post-transcriptional modification. In response to heat shock stress, increased methylation in the form of m6A promotes cap-independent translation initiation, providing a mechanism for selective mRNA translation under heat shock stress. Qian’s findings elucidate the physiological roles of m6A and uncovers a translational control mechanism in heat shock response.
Shu-Bing Qian, a 2009 New Innovator, demonstrated the dynamic nature of N6-methyladenosine (m6A) modification to mRNAs, the most abundant post-transcriptional modification. In response to heat shock stress, increased methylation in the form of m6A promotes cap-independent translation initiation, providing a mechanism for selective mRNA translation under heat shock stress. Qian’s findings elucidate the physiological roles of m6A and uncovers a translational control mechanism in heat shock response.
- Dynamic m6A mRNA Methylation Directs Translational Control of Heat Shock Response. Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Nature. 2015 Oct 22; 526(7574):591-4.
Imaging Gut Microbiota Organization and Interactions
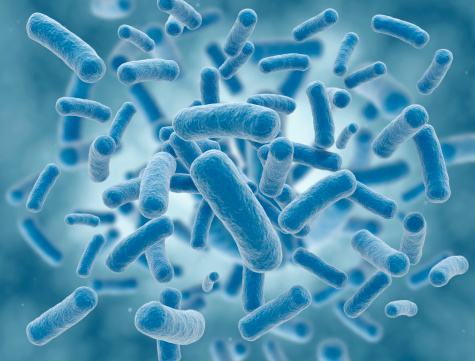 Justin Sonnenburg and Kerwyn Huang, 2009 New Innovators, developed a pipeline to assess the spatial organization and functional interactions of intestinal microbiota. Using immunofluorescence images of fixed gut cross-sections and BacSpace software, they created a method for high-throughput quantification of microbial organization.
Justin Sonnenburg and Kerwyn Huang, 2009 New Innovators, developed a pipeline to assess the spatial organization and functional interactions of intestinal microbiota. Using immunofluorescence images of fixed gut cross-sections and BacSpace software, they created a method for high-throughput quantification of microbial organization.
Quantitative Imaging of Gut Microbiota Spatial Organization. Earle KA et al. Cell Host Microbe. 2015 October 14; 18(4):478-88.
Bioengineering Lung Vasculature
 Harald Ott, a 2011 New Innovator, regenerated functional pulmonary vasculature by repopulating the vascular compartment of decellularized rat and human lungs scaffolds with human cells, including endothelial and perivascular cells derived from induced pluripotent stem cells. Increasing vascular performance will help maintain cell viability and improve function in lung grafts.
Harald Ott, a 2011 New Innovator, regenerated functional pulmonary vasculature by repopulating the vascular compartment of decellularized rat and human lungs scaffolds with human cells, including endothelial and perivascular cells derived from induced pluripotent stem cells. Increasing vascular performance will help maintain cell viability and improve function in lung grafts.
Engineering Pulmonary Vasculature in Decellularized Rat and Human Lungs. Ren X et al. Nat Biotechnol. 2015 October 8; 33(10):1097-102.
A Reference for Worldwide Human Genetic Variation
![]() Jeffrey Kidd, a 2011 Early Independence awardee, and Pardis Sabeti, a 2009 New Innovator, are part of the 1000 Genomes Project Consortium which released the findings from their project to whole-genome sequence a diverse set of individuals from multiple populations in Nature. In all, they sequenced the genomes of 2,604 individuals from 26 populations, discovering 88 million variants. Their resource includes >99% of SNP variants with a >1% frequency for a variety of ancestries. They discuss the distribution of genetic variation across the globe and discuss implications for common disease studies.
Jeffrey Kidd, a 2011 Early Independence awardee, and Pardis Sabeti, a 2009 New Innovator, are part of the 1000 Genomes Project Consortium which released the findings from their project to whole-genome sequence a diverse set of individuals from multiple populations in Nature. In all, they sequenced the genomes of 2,604 individuals from 26 populations, discovering 88 million variants. Their resource includes >99% of SNP variants with a >1% frequency for a variety of ancestries. They discuss the distribution of genetic variation across the globe and discuss implications for common disease studies.
- A Global Reference for Human Genetic Variation. 1000 Genomes Project Consortium et al. Nature. 2015 October 1; 526(7571):68-74.
3-Drug Combo Kills MRSA Superbug
 Gautam Dantas, a 2012 New Innovator, discovered a combination of three antibiotics is effective against Methicillin-resistant Staphylococcus aureus (MRSA). The three antibiotics are already approved by the FDA and are members of a broad class of drugs called beta-lactams, which are not effective against MRSA when used alone. Mice infected with MRSA died within a day, but infected mice treated with the triple antibiotics were completely cured. The study also found no evidence of antibiotic-resistance developing with the use of the combined antibiotics.
Gautam Dantas, a 2012 New Innovator, discovered a combination of three antibiotics is effective against Methicillin-resistant Staphylococcus aureus (MRSA). The three antibiotics are already approved by the FDA and are members of a broad class of drugs called beta-lactams, which are not effective against MRSA when used alone. Mice infected with MRSA died within a day, but infected mice treated with the triple antibiotics were completely cured. The study also found no evidence of antibiotic-resistance developing with the use of the combined antibiotics.
Synergistic, Collaterally Sensitive ß-Lactam Combinations Suppress Resistance in MRSA. Gonzales PR, et al. Nature Chemical Biology. 14 September 2015; 11:855-861.
Making Three-Dimensional Tissues
 Zev Gartner, a 2013 New Innovator, developed a method to create and program the size, shape, composition, and spatial heterogeneity of tissue. The method, called DNA-programmed assembly of cells (DPAC), uses cells with DNA tags that rapidly bind with complementary DNA sequence-tagged cells or substrates with high specificity. A DNA-patterned substrate functions as a template, and, layer-by-layer, DNA-tagged cells assemble to build 3D tissue structures. Once the structure is complete, extracellular matrix gels embed the tissue while DNase releases the tissue from the substrate. The method allows for the direction of specific cell types to precise locations in the 3D structure, as well as, dictating the size and shape of the tissue, and can generate tissue several centimeters long.
Zev Gartner, a 2013 New Innovator, developed a method to create and program the size, shape, composition, and spatial heterogeneity of tissue. The method, called DNA-programmed assembly of cells (DPAC), uses cells with DNA tags that rapidly bind with complementary DNA sequence-tagged cells or substrates with high specificity. A DNA-patterned substrate functions as a template, and, layer-by-layer, DNA-tagged cells assemble to build 3D tissue structures. Once the structure is complete, extracellular matrix gels embed the tissue while DNase releases the tissue from the substrate. The method allows for the direction of specific cell types to precise locations in the 3D structure, as well as, dictating the size and shape of the tissue, and can generate tissue several centimeters long.
Programmed Synthesis of Three-Dimensional Tissues. Todhunter ME, et al. Nature Methods. 31 August 2015; 12:975-981.
Engineering Bioartificial Limbs for Transplantation
 Harold Ott, a 2011 New Innovator, engineered a bioartificial limb graft for transplantation that reduces the risk of donor transplantation and the need for immunosuppression. Donor forearms from rats and primates were decellularized to create an acellular scaffold that preserved the composite architecture and properties of the musculoskeletal system. Muscle progenitor, endothelial, and mesenchymal cells were used to repopulate the scaffold and create a bio-composite graft. Bio-artificial grafts using the native scaffold and patient derived cells could be produced on demand and would not require immunosuppression after transplantation.
Harold Ott, a 2011 New Innovator, engineered a bioartificial limb graft for transplantation that reduces the risk of donor transplantation and the need for immunosuppression. Donor forearms from rats and primates were decellularized to create an acellular scaffold that preserved the composite architecture and properties of the musculoskeletal system. Muscle progenitor, endothelial, and mesenchymal cells were used to repopulate the scaffold and create a bio-composite graft. Bio-artificial grafts using the native scaffold and patient derived cells could be produced on demand and would not require immunosuppression after transplantation.
Engineered Composite Tissue as a Bioartificial Limb Graft. Jank BL, et al. Biomaterials. 2015 August; 61:246-256.
Anesthesia's Effects on the Brain Differ in the Elderly and in Children
 Patrick Purdon (2009 New Innovator) and Emery Brown (2012 Transformative Researcher and 2007 Pioneer) released four papers on brain activity under anesthesia in children and the elderly that address how to improve anesthesia care and advance the neuroscience of development and aging. They found anesthesia-induced brain dynamics mirrors brain development in children, where different brain wave patterns under anesthesia appear to “turn on” at particular ages, coinciding with known brain developmental milestones. A similar effect is seen in the elderly but in reverse—certain brain waves seem to fade away in a manner consistent with known patterns of brain aging. Elderly patients are much more sensitive to anesthetic drugs than current age-adjusted dosing guidelines suggest, and the elderly enter a coma-like state (associated with poor cognitive outcomes) at much lower doses than with young patients. Elderly animals emerge from anesthesia much more slowly than younger animals, but the drug methylphenidate accelerates emergence. Purdon and Brown also found commercially available EEG-based anesthetic monitors (developed for adults) seem inaccurate when applied to children and the elderly and suggest new age-specific monitoring guidelines.
Patrick Purdon (2009 New Innovator) and Emery Brown (2012 Transformative Researcher and 2007 Pioneer) released four papers on brain activity under anesthesia in children and the elderly that address how to improve anesthesia care and advance the neuroscience of development and aging. They found anesthesia-induced brain dynamics mirrors brain development in children, where different brain wave patterns under anesthesia appear to “turn on” at particular ages, coinciding with known brain developmental milestones. A similar effect is seen in the elderly but in reverse—certain brain waves seem to fade away in a manner consistent with known patterns of brain aging. Elderly patients are much more sensitive to anesthetic drugs than current age-adjusted dosing guidelines suggest, and the elderly enter a coma-like state (associated with poor cognitive outcomes) at much lower doses than with young patients. Elderly animals emerge from anesthesia much more slowly than younger animals, but the drug methylphenidate accelerates emergence. Purdon and Brown also found commercially available EEG-based anesthetic monitors (developed for adults) seem inaccurate when applied to children and the elderly and suggest new age-specific monitoring guidelines.
- Age-Dependent Electroencephalogram (EEG) Patterns During Sevoflurane General Anesthesia in Infants. Cornelissen L, Kim S-E, Purdon PL, Brown EN, and Berde CB. eLife. 2015 June 23; 4.e06513.
- The Ageing Brain: Age-Dependent Changes in the Electroencephalogram During Propofol and Sevoflurane General Anaesthesia. Purdon PL et al. Br J Anaesth. 2015 July; 115(Suppl 1):i46-i57.
- Ageing Delays Emergence from General Anaesthesia in Rats by Increasing Anaesthetic Sensitivity in the Brain. Chemali JJ et al. Br J Anaesth. 2015 July; 115(Suppl 1):i58-i65.
- Age-Dependency of Sevoflurane-Induced Electroencephalogram Dynamics in Children. Akeju O et al. Br J Anaesth. 2015 July; 115(Suppl 1):i66-i76.
SpeedSeq: Ultra-Fast Personal Genome Analysis and Interpretation
 Ira Hall, a 2009 New Innovator, published a paper in Nature Methods describing a new open-source genome analysis platform called SpeedSeq capable of aligning, detecting variants, and functionally annotating a 50x human genome in 13 hours on a low-cost server. SpeedSeq eliminates the substantial bioinformatics bottleneck that requires weeks of computation and extensive expert involvement.
Ira Hall, a 2009 New Innovator, published a paper in Nature Methods describing a new open-source genome analysis platform called SpeedSeq capable of aligning, detecting variants, and functionally annotating a 50x human genome in 13 hours on a low-cost server. SpeedSeq eliminates the substantial bioinformatics bottleneck that requires weeks of computation and extensive expert involvement.
SpeedSeq: Ultra-Fast Personal Genome Analysis and Interpretation. Chiang C et al. Nature. 2015 August 10.
Measuring the Wavefront of Scattered Light
 Changheui Yang, a 2010 New Innovator, published a paper in Nature Physics showing a new way to focus light in strong light-scattering media such as biological tissue. Controlling how light spreads through light-scattering media by shaping the light’s wavefront is challenging when the mapping between the input wavefront and scattered output wavefront is unknown. Yang overcame the mapping hurdle by using shift/shift optical correlations, rather than the traditional tilt/tilt correlations, allowing him to map the light’s wavefronts and opening new possibilities for communication and biomedical imaging.
Changheui Yang, a 2010 New Innovator, published a paper in Nature Physics showing a new way to focus light in strong light-scattering media such as biological tissue. Controlling how light spreads through light-scattering media by shaping the light’s wavefront is challenging when the mapping between the input wavefront and scattered output wavefront is unknown. Yang overcame the mapping hurdle by using shift/shift optical correlations, rather than the traditional tilt/tilt correlations, allowing him to map the light’s wavefronts and opening new possibilities for communication and biomedical imaging.
Translation Correlations in Anisotropically Scattering Media. Judkewitz B, Horstmeyer R, Vellekoop IM, Papadopoulos IN, and Yang C. Nature Physics. 2015 June 29; 11:684-689.
Reinforcing the Circadian Clock to Gate Immune Response
 Nicolas Buchler, a 2011 New Innovator, published a paper in Nature describing how two biological clocks work together to help plants maintain daily activities while dealing with intermittent demands, such as infections. The researchers identified the gene NPR1 in Arabidopsis as the master immune regulator, which acts as a sensor of the plant’s redox state and regulates transcription of core circadian clock genes. Changes in the redox status caused by immune challenges do not compromise the circadian clock but actually reinforce it by regulating morning and evening clock genes without changing the period. This balanced network helps plants gate immune responses toward morning and minimizes costs on growth at night.
Nicolas Buchler, a 2011 New Innovator, published a paper in Nature describing how two biological clocks work together to help plants maintain daily activities while dealing with intermittent demands, such as infections. The researchers identified the gene NPR1 in Arabidopsis as the master immune regulator, which acts as a sensor of the plant’s redox state and regulates transcription of core circadian clock genes. Changes in the redox status caused by immune challenges do not compromise the circadian clock but actually reinforce it by regulating morning and evening clock genes without changing the period. This balanced network helps plants gate immune responses toward morning and minimizes costs on growth at night.
Redox Rhythm Reinforces the Circadian Clock to Gate Immune Response. Zhou M et al. Nature. 2015 June 22; 523:472-476.
Accelerating Wound Healing
 Dino Di Carlo, a 2010 New Innovator, published a paper in Nature Materials showing accelerated wound healing with the use of an injectable, interconnected microporous gel scaffold from annealed microgel building blocks. The chemical and physical properties of the microgel building blocks can be tailored to fit the needs of the tissue and provides a scaffold for tissue regrowth and regeneration. In vitro, cells incorporated during the scaffold formation flourished and formed extensive three-dimensional networks within 48 hours. In vivo, the scaffolds aided cell migration that resulted in rapid tissue regeneration and structure formation within five days.
Dino Di Carlo, a 2010 New Innovator, published a paper in Nature Materials showing accelerated wound healing with the use of an injectable, interconnected microporous gel scaffold from annealed microgel building blocks. The chemical and physical properties of the microgel building blocks can be tailored to fit the needs of the tissue and provides a scaffold for tissue regrowth and regeneration. In vitro, cells incorporated during the scaffold formation flourished and formed extensive three-dimensional networks within 48 hours. In vivo, the scaffolds aided cell migration that resulted in rapid tissue regeneration and structure formation within five days.
Accelerated Wound Healing by Injectable Microporous Gel Scaffolds Assembled from Annealed Building Blocks. Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, and Segura T. Nature Materials. 2015 June 1; 14:737-744.
Resetting the Brain to Cure Developmental Disorders
 Sunil Gandhi, a 2013 New Innovator, published a paper in Neuron demonstrating a new method to reactivate plasticity in the adult brain to correct earlier developmental disorders through transplantation of embryonic inhibitory neurons. Using this method, Gandhi was able to correct amblyopia (lazy eye) in adult mice. The method is a powerful investigative tool for understanding developmental mechanisms that create critical periods in development and brain disorders. Such studies could lead to therapies for diseases like epilepsy, schizophrenia, and autism and could assist with conditions such as spinal cord injury or strokes.
Sunil Gandhi, a 2013 New Innovator, published a paper in Neuron demonstrating a new method to reactivate plasticity in the adult brain to correct earlier developmental disorders through transplantation of embryonic inhibitory neurons. Using this method, Gandhi was able to correct amblyopia (lazy eye) in adult mice. The method is a powerful investigative tool for understanding developmental mechanisms that create critical periods in development and brain disorders. Such studies could lead to therapies for diseases like epilepsy, schizophrenia, and autism and could assist with conditions such as spinal cord injury or strokes.
Inhibitory Neuron Transplantation into Adult Visual Cortex Creates a New Critical Period that Rescues Impaired Vision. Davis MF et al. Neuron. 2015 May 20; 86(4):1055-1066.
Scientists Cure Disorders in Mice by Resetting Their Brains
Monitoring the Progress of Stem Cells after Transplantation into Brain
 Jin Hyung Lee (2010 awardee) devised a way to monitor neural stem cells after transplantation in the brain by combining functional magnetic resonance imaging, optogenetics, and electrophysiology. Many central nervous system disorders, such as Parkinson’s disease, are characterized by defective nerve cells in specific brain regions, making them excellent candidates for stem cell therapies, which replace defective nerve cells. However, assessing the success of such experiments are difficult because there is no good way to evaluate what happens to transplanted stems cells following the procedure. With Lee’s new method, scientists are able to determine not only whether the stem cells transplanted into living animals survived, but also whether they matured into nerve cells, integrated into targeted brain circuits, and function correctly. Being able to monitor stem cell transplantations will help guide future therapies—what kind of cells to put in, where to place them, and how—and will accelerate the development of new treatments of central nervous system disorders.
Jin Hyung Lee (2010 awardee) devised a way to monitor neural stem cells after transplantation in the brain by combining functional magnetic resonance imaging, optogenetics, and electrophysiology. Many central nervous system disorders, such as Parkinson’s disease, are characterized by defective nerve cells in specific brain regions, making them excellent candidates for stem cell therapies, which replace defective nerve cells. However, assessing the success of such experiments are difficult because there is no good way to evaluate what happens to transplanted stems cells following the procedure. With Lee’s new method, scientists are able to determine not only whether the stem cells transplanted into living animals survived, but also whether they matured into nerve cells, integrated into targeted brain circuits, and function correctly. Being able to monitor stem cell transplantations will help guide future therapies—what kind of cells to put in, where to place them, and how—and will accelerate the development of new treatments of central nervous system disorders.
Direct in vivo assessment of human stem cell graft-host neural circuits. Byers B et al. Neuroimage. 2015 Apr 22; http:// dx.doi.org/10.1016/j.neuroimage.2015.03.079.
Manipulating Gene Regulation
 Charles Gersbach, a 2011 awardee, developed a new tool for manipulating gene regulation. By creating and using a programmable CRISPR-Cas9-based-acetyltransferase, Gersbach and colleagues demonstrate it is possible to activate transcription of target genes from promoters and proximal and distal enhancers with high specificity. This new tool will enable direct manipulation of the epigenome and downstream regulation, allowing researchers to examine the relationship between the epigenome and transcriptional control with the potential of precisely controlling cell phenotypes.
Charles Gersbach, a 2011 awardee, developed a new tool for manipulating gene regulation. By creating and using a programmable CRISPR-Cas9-based-acetyltransferase, Gersbach and colleagues demonstrate it is possible to activate transcription of target genes from promoters and proximal and distal enhancers with high specificity. This new tool will enable direct manipulation of the epigenome and downstream regulation, allowing researchers to examine the relationship between the epigenome and transcriptional control with the potential of precisely controlling cell phenotypes.
Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Hilton IB et al. Nat Biotechnol. 2015 May; 33(5):510-517.
HIV Latency Confers Evolutionary Advantage
 Leor Weinberger, 2013 Pioneer and 2009 New Innovator Awardee, published back-to-back papers in Cell that demonstrate HIV latency is caused by the virus itself, rather than by the immune cells as was previously thought, and identified the protein Tat as the crucial regulator of mediating whether the virus is active or latent. This work also suggests that latency is a naturally selected tactic employed by the virus to increase infection rates. This research provides important information about how and why latency is established and maintained, opening the door to novel therapeutic approaches to treat HIV infection.
Leor Weinberger, 2013 Pioneer and 2009 New Innovator Awardee, published back-to-back papers in Cell that demonstrate HIV latency is caused by the virus itself, rather than by the immune cells as was previously thought, and identified the protein Tat as the crucial regulator of mediating whether the virus is active or latent. This work also suggests that latency is a naturally selected tactic employed by the virus to increase infection rates. This research provides important information about how and why latency is established and maintained, opening the door to novel therapeutic approaches to treat HIV infection.
A hardwired HIV latency program. Razooky BS et al. Cell. 2015 Feb 26; 160(5):990-1001.
An evolutionary role for HIV latency in enhancing viral transmission. Rouzine IM, Weinberger AD, Weinberger LS. Cell. 2015 Feb 26; 160(5):1002-1012.
Protein Tangles Spell Trouble for Cancer Cells
 New research has demonstrated that a destructive biological process involved in Alzheimer’s disease may hold promise as a novel treatment strategy for cancer. Anomalies in protein folding can generate harmful protein deposits within cells, called amyloids. These amyloid deposits underlie several neurodegenerative disorders in humans, particularly Alzheimer's disease. While amyloids are widely recognized as killers of neurons, a new study from 2010 awardee Chengkai Dai at The Jackson Laboratory reveals that cancer cells are also vulnerable to these toxic proteins. Cancer cells manage to contain deleterious amyloids by hijacking a cellular stress response that has evolved to counter protein misfolding, thereby sustaining the cancer cells’ vicious survival and growth. But importantly, this study also points to a possible means of countering this effect. Surprisingly, the authors’ findings reveal that cancer cells, unlike normal cells, constantly suffer heightened intrinsic stress. As a result, cancer cells are therefore particularly susceptible to extrinsic insults, including the activity of therapeutic agents that either perturb protein folding or subdue the defensive stress response. These agents efficiently induce the generation of amyloids within cancer cells and thereby exert potent anti-cancer effects. Thus, this study highlights a potential therapeutic strategy that harnesses the culprit behind neurodegenerative diseases to combat malignancy.
New research has demonstrated that a destructive biological process involved in Alzheimer’s disease may hold promise as a novel treatment strategy for cancer. Anomalies in protein folding can generate harmful protein deposits within cells, called amyloids. These amyloid deposits underlie several neurodegenerative disorders in humans, particularly Alzheimer's disease. While amyloids are widely recognized as killers of neurons, a new study from 2010 awardee Chengkai Dai at The Jackson Laboratory reveals that cancer cells are also vulnerable to these toxic proteins. Cancer cells manage to contain deleterious amyloids by hijacking a cellular stress response that has evolved to counter protein misfolding, thereby sustaining the cancer cells’ vicious survival and growth. But importantly, this study also points to a possible means of countering this effect. Surprisingly, the authors’ findings reveal that cancer cells, unlike normal cells, constantly suffer heightened intrinsic stress. As a result, cancer cells are therefore particularly susceptible to extrinsic insults, including the activity of therapeutic agents that either perturb protein folding or subdue the defensive stress response. These agents efficiently induce the generation of amyloids within cancer cells and thereby exert potent anti-cancer effects. Thus, this study highlights a potential therapeutic strategy that harnesses the culprit behind neurodegenerative diseases to combat malignancy.
MEK guards proteome stability and inhibits tumor-suppressive amyloidogenesis via HSF-1. Tang et al. Cell. 2015 Feb 12; 160(4):729-744.
Single Animal to Human Transmission Event Responsible for 2014 Ebola Outbreak
 Pardis Sabeti, senior associate member of the Broad Institute and 2009 awardee, led an extensive analysis of the genetic makeup of Ebola samples from patients living in affected regions. The international team of scientists used advanced technology to analyze the genetics of the Ebola samples extremely rapidly and were able to pinpoint a single animal human transmission event.
Pardis Sabeti, senior associate member of the Broad Institute and 2009 awardee, led an extensive analysis of the genetic makeup of Ebola samples from patients living in affected regions. The international team of scientists used advanced technology to analyze the genetics of the Ebola samples extremely rapidly and were able to pinpoint a single animal human transmission event.
Two NIH Common Fund High Risk – High Reward Researchers named as 2013 Allen Distinguished Investigators

 The Paul G. Allen Family Foundation, co-founded by Microsoft co-founder Paul G. Allen and his sister Jo Lynn Allen, was created with the goal of “transforming lives and strengthening communities by fostering innovation, creating knowledge, and promoting social progress” through its philanthropic efforts. In 2010, the Foundation created the Allen Distinguished Investigators Awards to support high-risk, high-reward ideas in science. The program awards grants to ambitious scientists who are pioneering, innovative, early-stage researchers. Two NIH High-Risk, High-Reward Investigators were selected as 2013 Allen Distinguished Investigators: Jeff Gore (2012 New Innovator from MIT) and Markus Covert (2009 Pioneer from Stanford University). Gore will use the $1.5 million prize to investigate decision making and the evolutionary origins or cooperation using yeast as a model. Covert will also receive $1.5 million and plans to use this grant to study whole-cell models of higher organisms, including human cells. These awards will be funded over the course of three years, and the Foundation hopes these grants will enable the awardees to unlock fundamental questions in biology that are essential to achieving world-changing breakthroughs.
The Paul G. Allen Family Foundation, co-founded by Microsoft co-founder Paul G. Allen and his sister Jo Lynn Allen, was created with the goal of “transforming lives and strengthening communities by fostering innovation, creating knowledge, and promoting social progress” through its philanthropic efforts. In 2010, the Foundation created the Allen Distinguished Investigators Awards to support high-risk, high-reward ideas in science. The program awards grants to ambitious scientists who are pioneering, innovative, early-stage researchers. Two NIH High-Risk, High-Reward Investigators were selected as 2013 Allen Distinguished Investigators: Jeff Gore (2012 New Innovator from MIT) and Markus Covert (2009 Pioneer from Stanford University). Gore will use the $1.5 million prize to investigate decision making and the evolutionary origins or cooperation using yeast as a model. Covert will also receive $1.5 million and plans to use this grant to study whole-cell models of higher organisms, including human cells. These awards will be funded over the course of three years, and the Foundation hopes these grants will enable the awardees to unlock fundamental questions in biology that are essential to achieving world-changing breakthroughs.
The Allen Distinguished Investigators Award
Two HRHR researchers awarded world’s largest brain research prize

 Two NIH Common Fund High-Risk, High-Reward Awardees were named as winners of The Brain Prize for 2013, which is awarded by the Grete Lundbeck European Brain Research Foundation. Americans, Karl Deisseroth, a 2005 Pioneer Awardee, and 2012 Transformative Research Awardee, and Edward S. Boyden, a 2007 New Innovator, and 2012 Transformative Research Awardee and four European scientists were awarded the 2013 prize for their contributions to the development of “optogenetics.” Optogenetics is a technique that merges the fields of optics and genetics using light to specifically control the activity of genetically selected neurons. This revolutionary technique has allowed scientists to begin to gain a better understanding of the way circuits of neurons carry out complex functions. This technique has broad application potential in research from studying fundamental activities, such as memory formation and breathing, to more complex disorders and diseases, such as addiction, psychiatric disorders, and Alzheimer’s disease.
Two NIH Common Fund High-Risk, High-Reward Awardees were named as winners of The Brain Prize for 2013, which is awarded by the Grete Lundbeck European Brain Research Foundation. Americans, Karl Deisseroth, a 2005 Pioneer Awardee, and 2012 Transformative Research Awardee, and Edward S. Boyden, a 2007 New Innovator, and 2012 Transformative Research Awardee and four European scientists were awarded the 2013 prize for their contributions to the development of “optogenetics.” Optogenetics is a technique that merges the fields of optics and genetics using light to specifically control the activity of genetically selected neurons. This revolutionary technique has allowed scientists to begin to gain a better understanding of the way circuits of neurons carry out complex functions. This technique has broad application potential in research from studying fundamental activities, such as memory formation and breathing, to more complex disorders and diseases, such as addiction, psychiatric disorders, and Alzheimer’s disease.
The Brain Prize, the world’s largest brain research prize, is awarded to scientists who have made an outstanding contribution to European brain research, and is awarded to scientists who have conducted research in Europe, or to scientists who have conducted research in collaboration with European scientists.
Brain Prize
2013 Press Release .
Scientists Bioengineer Transplantable Kidney In Rats
New Innovator Harald C. Ott and his colleagues at Massachusetts General Hospital (MGH) have developed a new method to bioengineer kidneys that could someday enable scientists to generate and transplant patient-specific bioengineered organs into patients. Kidneys play a critical role in the maintenance of homeostasis in the body by filtering waste and excess fluid from the blood. There are approximately one million patients in the U.S. with kidney failure, or end-stage renal disease (ESRD); the only possible cure is kidney transplantation. Currently, the waitlist for kidney transplants in the U.S. is more than three years long. Even with successful transplantation there is a high risk for transplant rejection or loss of transplant function over time.
The scientists from MGH were able to remove cells from cadaver kidneys, not normally suitable for transplantation, to create a cell-free kidney scaffold that maintained the protein structure and composition of normal functioning kidneys. Once the host cells were removed, the researchers used human umbilical vein cells and neonate mice kidney cells to repopulate the kidney. Following an incubation that mimics the human body environment, the researchers showed that the patient-specific kidneys had restored function and were able to reabsorb electrolytes and sugars, and produce urine both in the laboratory and in kidneys they transplanted into rats. These bioengineered kidneys could potentially address two major issues currently challenging kidney transplantations. First, they provide the initial steps towards developing kidneys in the lab that can be transplanted into patients, effectively reducing the wait time for critical ESRD patients. Second, developing the technology to repopulate bioengineered kidneys with patient-specific cells could result in a reduction in the risk of kidney rejection after transplantation. While these bioengineered kidneys still need to be developed further before they become a fully implantable treatment option in human patients, this new technology can be applied to other organs, and is leading the way toward organ bioengineering.
- Watch Dr. Ott describe this work on the NatureVideoChannel here
- Read the MGH News Release here
Researchers Define Precise EEG Signature of Anesthesia-Induced Unconsciousness

More than 60,000 patients in the United States undergo general anesthesia for surgery every day. Currently, doctors use indirect measures of the brain state, such as heart rate and blood pressure, along with drug pharmacokinetics, pharmacodynamics and exhaled anesthetic gas typically are converted to a simple index score to indicate if a patient is adequately anesthetized during surgery. These indices are neither entirely reliable nor very informative. Inadequately administered anesthesia can result in intraoperative awareness and post-operative delirium. Drs. Emery N. Brown and Patrick L. Purdon, former NIH Director’s Pioneer Awardee and current NIH Director’s New Innovator Awardee, respectively, have studied brain activity during propofol-induced anesthesia using electroencephalogram (EEG) technology, which measures scalp electrical potentials, to better understand the mechanism of unconsciousness and to establish a direct method of tracking the transition between consciousness and unconsciousness during general anesthesia. In this study, the researchers gradually administered and reduced the anesthesia drug propofol in patients, while monitoring brain activity and behavioral loss of consciousness by asking patients to respond to auditory stimuli. This gradual induction and emergence from anesthesia allowed the researchers to precisely define an EEG brain signature, as well as behavioral markers that are associated with consciousness, unconsciousness, and the transition between these two states in patients undergoing propofol-induced general anesthesia. This research has provided a deeper understanding of the mechanism of propofol-induced loss of consciousness, and could be used to determine the EEG brain signatures of other anesthetics. Additionally, these results could be used to develop more direct and reliable methods for monitoring brain states of patients undergoing general anesthesia and could lead to new insights into the tailoring of drug dosage in real-time.
Success with Gene Circuits Could Lead to New Approaches to Gene Therapy

A gene circuit refers to the complex mechanism by which genetic material, encoded by DNA, interacts with proteins resulting in a specific phenotype, or an observable characteristic. This can be more readily understood by imagining an electrical circuit, where you can turn a light switch on or off to produce an observable characteristic of light or darkness, respectively. Gene circuits can be turned on or off just like a switch, thus the expression of the associated phenotype can therefore be turned off or on. Additionally, these circuits can be expressed as more than just on or off. Imagine a light switch with a dimming function by which the light can be expressed at different intensities. Similarly, these gene circuits can be regulated in order to express at different intensities rather than just on or off, in order to produce phenotypic differences at varying levels of expression. In nature, multiple genes in multiple gene circuits of a cell interact to regulate major biological processes like cell signaling or division. Researchers can construct simple synthetic gene circuits in order to study the expression of specific genes in living systems, such as microorganisms, as well as to create novel biological functions for practical applications.
NIH Director’s New Innovator Awardee, Gábor Balázsi, and colleagues have described a new method where they developed and optimized a synthetic gene circuit in yeast and were able to adapt the gene circuit to mammalian cells. Importantly, the authors optimized this gene circuit allowing them to finely adjust gene expression in a linear fashion with uniform gene expression across the entire cell population. Previously, scientists had been unable to directly transfer gene circuits developed in microorganisms directly into mammalian systems, such as human cells. Dr. Balázsi’s new findings will allow researchers to design circuits that can be tested and characterized in microorganisms, such as yeast, which are easier to engineer than mammalian cells, and then introduce the circuits into mammalian cells, a model more similar and representative to study the complexities of human disease. These gene circuits can be used to study the effects of specific gene expression on biological functions such as development, immune response, and nervous system response, and can be used to develop precisely controlled gene expression systems for use in individualized gene therapy to treat specific diseases and genetic conditions.
New Model Allows Scientists to Study Mechanism of HCM Development and Test Drugs for Treatment
Hypertrophic Cardiomyopathy (HCM) is a prevalent heart condition that involves the thickening of the heart walls, which overtime can lead to the development of an irregular heart beat and sudden cardiac death. HCM has been estimated to be the most common inherited heart condition in the world, and has been associated with several DNA mutations affecting sarcomeres, or the basic unit of a muscle.
Dr. Joseph Wu, an NIH Director’s New Innovator Award Recipient from Stanford University, and his colleagues, have developed a new technology using induced pluripotent stem cells (iPSCs) obtained from skin cells of a family of patients that allows them to model HCM at the level of a single cell. The researchers were able to turn the skin-derived iPSCs into heart muscle cells, permitting them to investigate the underlying causes of HCM development without requiring the difficult direct collection of diseased heart tissue. This model allowed the researchers to better understand how a specific DNA mutation, carried by affected members of this particular family, can lead to the development of HCM, and helped them to test therapies that might be used to treat or prevent HCM due to this specific mutation. This new technology can be used by other researchers to investigate the effect of other DNA mutations on the development of HCM, and to screen the effectiveness of new treatments and preventative therapies specific to each mutation.
Read the San Francisco Chronicle Article...
Self Assembling DNA Bricks

Dr Peng Yin and Dr. William Shih, both awardees of the Common Fund NIH Director’s New Innovator Award program, have described a new method to construct complex three dimensional structures by using short synthetic DNA strands which the investigators call “DNA bricks.” In this paper, the investigators used 32-nucleotide DNA strands which self-assembled into prescribed 3D structures without scaffolds. Each DNA brick can bind up to four local neighbors, and in a manner similar to Lego® bricks can be used to build complicated structures. This self assembly has allowed the investigators to create over 100 distinct structures which can be found here. This method may have a large variety of applications such as to develop smart drug delivery particles or provide a better way for nano-scale fabrication of inorganic materials.
More information describing the self assembly process can be found in the two videos below:
Video 1 Animated video containing narration which describes how 3D structures of DNA bricks are assembled.
Video 2 Animated video which depicts the nanofabrication technique called “DNA-brick self-assembly” using short synthetic strands of DNA that work like interlocking Lego® bricks.
Tele-medical Device Wins Top Honors

Dr. Aydogan Ozcan, an NIH Director’s New Innovator Award recipient, and colleagues at the University of California, Los Angeles (UCLA) garner first place in The Scientist magazine, “Top Ten Innovations of 2011” competition by developing a cell phone camera attachment that functions as a mobile microscope. The device known as LUCAS (Lensless, Ultra-wide-field Cell monitoring Array platform based on Shadow imaging), is a light-weight holographic microscope that uses inexpensive off-the-shelf parts as an attachment to a cell phone’s camera. Biospeciman samples are loaded into the slide attachment of the device, and then battery operated light emitting diodes (LEDs) are used to illuminate the specimen and produce an image of the cells shadows. The image is then remastered using an algorithm remotely run from a computer. Although the resolution of the LUCAS is at the submicrometer level, the microscope can be used for wide-field imaging. The technology coupled with the use of a cell phone provides a plethora of opportunities to capture and electronically transport biospeciman images in real time. The device can be used for water quality testing and even to monitor diseases such as Malaria. At the estimated cost of approximately 10 USD for the LUCAS attachment, the technology has the potential of providing diagnostic services to resource limited areas across the nation. Field tests around the world are already being planned for the LUCAS in 2012.

New Material Holds Promise for Drug Delivery, Medical Implants
Dr. Adah Almutairi, a 2009 NIH Director’s New Innovator awardee, and colleagues at the University of California San Diego, have developed a new type of “smart” polymeric material that may have widespread medical and biological applications. Reported in the journal Macromolecules, the new material disassembles in response to harmless levels of near infrared (NIR) irradiation, which can penetrate up to 10 centimeters (almost 4 inches) into the body. Both the material itself and its breakdown products are well-tolerated by living cells, suggesting this material would potentially be safe for use in humans. This type of material could be used as a capsule for a drug, allowing doctors to only release the drug in a specific area, such as right next to a tumor. It could also be used in tissue engineering, implants, wound-healing, and biosensors. Dr. Almutairi and colleagues are now working on improving the design of this polymeric material, so that it becomes even more sensitive to NIR, allowing a more controlled disassembly of the material. This research is a significant step forward in the development of light-sensitive materials that will allow doctors and researchers to target previously inaccessible sites with precise spatial and temporal control.
Location, Location, Location: Scientists Uncover New Information about Brain Stem Cell Environment
The adult mammalian brain contains several specialized areas where stem cells capable of producing new neurons reside. Dr. Chay Kuo, an NIH Director’s New Innovator Award recipient, has identified key components of one such specialized area, or niche, that is critical for the production of new neurons. Published in the July 14, 2011 issue of Neuron, Dr. Kuo and colleagues demonstrate that two proteins, Foxj1 and Ank3, are critical for maintaining the cellular niche around brain stem cells. Foxj1 is a transcription factor, a type of protein that regulates when and where other genes are expressed. Within the stem cell niche studied by Dr. Kuo, the Foxj1 protein causes the Ank3 protein to be expressed. The Ank3 protein then helps assemble groups of ependymal cells, a type of cell that surrounds the brain stem cells and provides support for the production of new neurons. Without Foxj1 and Ank3 proteins, the niche is disrupted and stem cells fail to produce new neurons. Currently, when neural stem cells are studied in the laboratory, they are not surrounded by these ependymal cells. Under these conditions, it is extremely difficult to make the stem cells produce neurons. The findings of Dr. Kuo and colleagues suggests that in the laboratory setting, scientists may need to recreate the same kind of support provided by ependymal cells in the brain stem cell niche. By understanding the local cellular environment that helps support production of new neurons, researchers hope to improve future therapeutic strategies that use stem cells to produce neurons for repairing or replacing damaged tissue.
High Throughput Strategy for Testing Nerve Regeneration
 Finding new drugs to promote regeneration of damaged nerve cells holds great promise for diseases such as Alzheimer's disease, spinal cord injury, brain trauma, and more. Many potential treatments, while promising in cell cultures, fail to promote regeneration in living animals. In a paper published October 13th in the Early Edition of the Proceedings of the National Academy of Sciences, Dr. Mehmet Yanik, a researcher at the Massachusetts Institute of Technology and an NIH Director's New Innovator awardee, demonstrates a novel method to rapidly screen potential drugs for their ability to promote nerve regeneration in the nematode C. elegans. Using this method, Dr. Yanik and colleagues discovered that compounds which regulate protein kinase C (PKC), an enzyme important for many different cellular processes, can modulate nerve regeneration after injury in specific neurons. The ability of this method to efficiently screen large numbers of potential drugs in living animals may greatly accelerate the discovery of new treatments to promote nerve regeneration.
Finding new drugs to promote regeneration of damaged nerve cells holds great promise for diseases such as Alzheimer's disease, spinal cord injury, brain trauma, and more. Many potential treatments, while promising in cell cultures, fail to promote regeneration in living animals. In a paper published October 13th in the Early Edition of the Proceedings of the National Academy of Sciences, Dr. Mehmet Yanik, a researcher at the Massachusetts Institute of Technology and an NIH Director's New Innovator awardee, demonstrates a novel method to rapidly screen potential drugs for their ability to promote nerve regeneration in the nematode C. elegans. Using this method, Dr. Yanik and colleagues discovered that compounds which regulate protein kinase C (PKC), an enzyme important for many different cellular processes, can modulate nerve regeneration after injury in specific neurons. The ability of this method to efficiently screen large numbers of potential drugs in living animals may greatly accelerate the discovery of new treatments to promote nerve regeneration.
New Tools to Correct Brain Activity
 Many neurological and psychiatric disorders are associated with abnormal activity in specific brain circuits. Historical approaches to correct abnormalities in brain circuits have relied on the use of electrical or magnetic stimulation, which only relieve the symptoms partially or for a short time. Although contemporary electromagnetic stimulation techniques have overcome many of the drawbacks of earlier approaches, a continuing and pressing need remains for new medical approaches to systematically correct abnormal brain function in patients with epilepsy, brain injury, and Parkinson’s disease.
Many neurological and psychiatric disorders are associated with abnormal activity in specific brain circuits. Historical approaches to correct abnormalities in brain circuits have relied on the use of electrical or magnetic stimulation, which only relieve the symptoms partially or for a short time. Although contemporary electromagnetic stimulation techniques have overcome many of the drawbacks of earlier approaches, a continuing and pressing need remains for new medical approaches to systematically correct abnormal brain function in patients with epilepsy, brain injury, and Parkinson’s disease.
Dr. Ed Boyden in the Common Fund’s New Innovator program has engineered a powerful new class of tools to shut down nerve activity for short periods of time using different colors of light. These techniques are based on genes recovered from bacteria and fungi that encode light-activated proteins normally used for energy production in these organisms. When nerve cells expressing these proteins are exposed to the appropriate wavelength of light, they are prevented from transmitting electrical signals. When used in combination with genetic techniques to target these proteins to specific brain regions or cell subsets, these tools can lead to a much deeper understanding of the brain’s role in health and disease. The development of new technologies that allow precise control of neural circuits could lead to new treatments for disorders associated with abnormal brain activity, including chronic pain, epilepsy, brain injury, and Parkinson’s disease.



