Scientists have uncovered striking new details about tiny cellular “gatekeepers” that play major roles in inflammation, hearing, and a wide range of diseases. These insights could pave the way toward developing precise, next-generation drugs for better health.
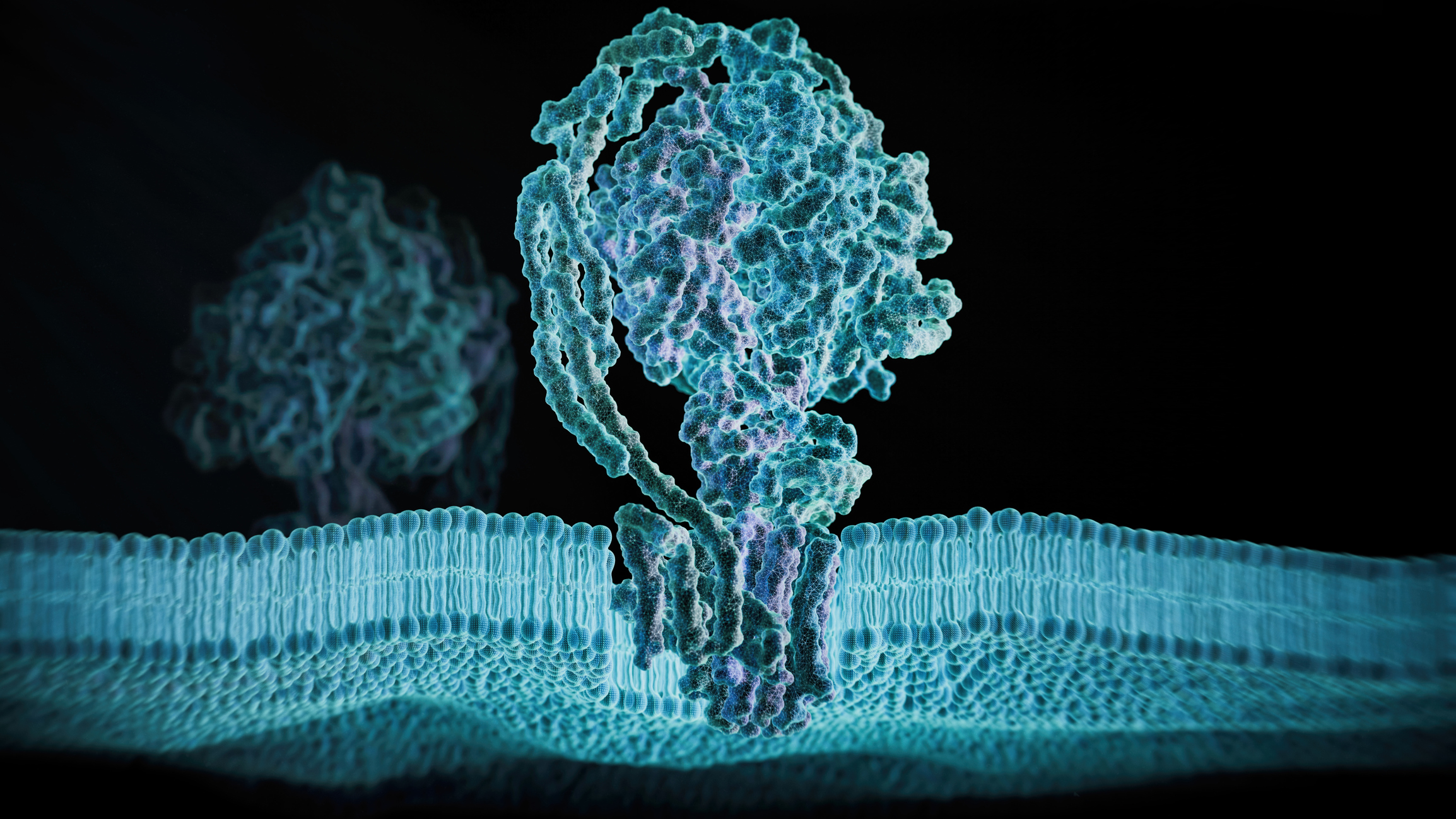
Adenosine triphosphate (ATP) powers nearly all cell activity. When cells are stressed or injured, ATP can leak out and act as a distress signal. P2X receptors – gate-like proteins on the cell surface – sense this ATP and trigger biological responses. Sometimes, these responses can help the cells heal. But in other cases, these responses can lead to chronic inflammation, pain disorders, hearing loss, and diseases such as Alzheimer’s and heart disease.
Scientists still do not fully understand how this happens, though deciphering this puzzle is pivotal for developing drugs to adjust how the receptors work. However, progress has been limited because the scientific community does not have detailed knowledge about the receptors’ structure and how they function at the molecular level.
High-Risk, High-Reward Research (HRHR) NIH Director's New Innovator Award recipient Steven Mansoor, M.D., Ph.D., at Oregon Health & Science University, set out to tackle this challenge. Mansoor and his research team examined the molecular structure of one of the human P2X receptors: P2X7R. P2X7R is a protein that triggers inflammation and plays a role in diseases such as cancer, heart disease, and neurodegenerative disorders like Alzheimer’s Disease. Although blocking P2X7R could help treat these diseases, no approved drugs currently target this receptor. To address this, Dr. Mansoor’s team first used cryo-electron microscopy (cryo-EM) to develop high-resolution, 3D images of the human P2X7R. By comparing these images using computer simulations, the researchers then designed a new and highly targeted experimental drug called UB-MBX-46. This compound attaches to a specific site on the receptor, effectively blocking activity. UB-MBX-46 shows great potential as a treatment for inflammation and could eventually be developed to help a wide range of diseases where inflammation plays a major role.
The researchers also studied a related human P2X receptor: P2X2R, involved in normal hearing. To address the limited knowledge of this receptor protein, Dr. Mansoor’s team again used cryo-EM to capture detailed 3D images of the human P2X2R. They discovered unique structural features and located specific sites, that when mutated, result in hearing defects. The team also revealed how ATP binds to and reshapes P2X2R, identifying two distinct inactive forms – one of which is novel. These findings give researchers a much better understanding of how P2X2R functions and may provide useful information for developing new treatments to prevent hearing loss linked to this receptor.
Dr. Mansoor’s research represents a breakthrough in uncovering P2X receptors’ role in inflammation and affecting the body’s sensory systems. By revealing these receptors’ detailed structures, researchers have paved the way toward developing precise, next-generation drugs that could target and treat a wide range of diseases.
References:
Westermann, F. G., Oken, A. C., Granith, P. K. E., Marimuthu, P., Müller, C. E., & Mansoor, S. E. (2025). Subtype-specific structural features of the hearing loss–associated human P2X2 receptor. Proceedings of the National Academy of Sciences of the United States of America, 122(37), e2417753122. https://doi.org/10.1073/pnas.2417753122
Oken, A. C., Turcu, A. L., Tzortzini, E., Westermann, F. G., & Mansoor, S. E. (2025). A polycyclic scaffold identified by structure-based drug design effectively inhibits the human P2X7 receptor. Nature Communications, 16, 8283. https://doi.org/10.1038/s41467-025-62643-8



