What if we could reverse the impact of a disease at the genetic level? Essentially, restoring cells in the body to their normal function. This is a question that genomic researchers in scientific laboratories across the globe are trying to answer. Within the last decade, a technology called CRISPR-Cas9 has been leveraged with this intent. CRISPR was first discovered as part of a natural genome editing system bacteria use as an immune defense. The other component of the system, the Cas9 protein, cuts invading viral DNA. Because the system can recognize and act on specific genetic sequences in a variety of cell types, including human cells, scientists have tweaked the CRISPR-Cas9 system to successfully replace faulty genes with healthy ones. There have been promising discoveries in animal models and ex vivo studies (where a gene is manipulated outside of a living organism, usually in cells grown in the lab). Scientists continue to work to harness the full potential of CRISPR-Cas9 to treat an array of medical conditions. In fact, in some instances CRISPR Cas9 is being used as a treatment option against select diseases.
However, manipulating the human genome and calculating for individual variability requires precision. Even minor miscalculations could have significant impacts and unintended adverse outcomes. For example, Cas9 sometimes significantly disrupts or removes portions of a target gene. In cases where editing relies on precise insertion of a new DNA sequence, this disruption is unhelpful.
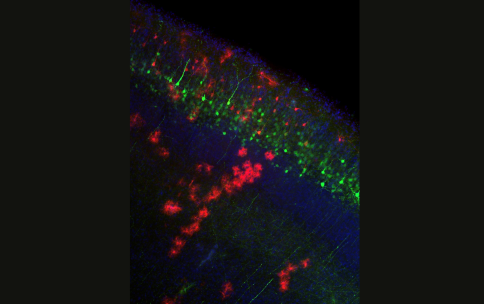
Dr. Alexandros Poulopoulos, a recipient of the 2019 NIH Director’s New Innovator Award from the Common Fund’s High-Risk High-Reward Research program, and colleagues have developed a new workflow to increase the precision of gene editing and reduce unintended disruption of target genes. To do this, the team screened various groupings of DNA, Cas9, and other DNA repair proteins for a combination that would create an editing system meeting their dual criteria of efficiency and precision. According to Dr. Poulopoulos, the team identified Cas9-RC, a new version of Cas9 designed for genome editing in the brain. Cas9-RC, is a fusion of Cas9 with two other DNA repair proteins. This novel combination not only met the researchers’ dual criteria but was found to increase the performance of gene editing both in cells grown in the lab and in the cells of mouse brains. While Dr. Poulopoulos and colleagues note that more work still needs to be done, the study team hopes that this improved workflow will help to inform future genomic therapies.
Full details about their study are published in the CRISPR journal.
Read more
Enhancing precision and efficacy of Cas0-mediated knockin through combinatorial fusions of DNA repair proteins. Richardson, R, Steyert M, Khim, SN, Crutcher GW, Brandenburg C, Robertson CD, Romanowski AJ, Inen, J, Altas B, Poulopoulos, A. The CRISPR Journal, 2023 Oct; 6(5): 447-461.
doi: 10.1089/crispr.2023.0036.
References
What Is CRISPR, and Why Is It So Important? Tabb, M, Gawrylewski, A, DelViscio, J. Scientific American, 2021 June.
Off-target effects in CRISPR/Cas9 gene editing. Guo C, Ma X, Gao F, Guo Y. Front Bioeng Biotechnol. 2023 Mar 9; 11:1143157. doi: 10.3389/fbioe.2023.1143157. PMID: 36970624; PMCID: PMC10034092.



