Science Highlights Archives
Dynamic DNA Loops Affect How Cells Become Specialized
Genomic DNA is packaged and organized in the tiny nucleus of the cell as chromatin  (a complex of DNA and histone proteins). The 3-dimensional organization of chromatin in the nucleus affects which genes are expressed and at what times. A complex network of chromatin loops is involved in coordinating changes in transcription during cell development. Chromatin loops can bring enhancers (sections of DNA that promote transcription when bound by proteins called transcription factors) closer to their target genes in the genome. How chromatin architecture changes as cells differentiate into specialized cell types and how these changes affect cell-type-specific gene expression and cellular function are not well understood.
(a complex of DNA and histone proteins). The 3-dimensional organization of chromatin in the nucleus affects which genes are expressed and at what times. A complex network of chromatin loops is involved in coordinating changes in transcription during cell development. Chromatin loops can bring enhancers (sections of DNA that promote transcription when bound by proteins called transcription factors) closer to their target genes in the genome. How chromatin architecture changes as cells differentiate into specialized cell types and how these changes affect cell-type-specific gene expression and cellular function are not well understood.
A team of researchers, including 4DN program-funded researcher Erez Lieberman Aiden, used a technique called in situ chromosome conformation capture (Hi-C) to create high-resolution genome-wide looping maps to compare the chromatin structure of cells before and after differentiation to become specialized immune cells (macrophages). Hi-C is a method of detecting frequencies of contact between all mappable regions of the human genome. Following differentiation, they found genes at loops that were newly formed (“gained loops”) or newly activated by changes in chromatin architecture (“activated loops”) have increased expression. The gained and activated loops form multi-loop activation “hubs” that create long-range interactions between active enhancers and promoters and have increased binding of transcriptional regulators, thus facilitating transcription. The multi-loop hubs occur at genes known to play a role in macrophage development and function, indicating a role in regulating gene transcription during cell differentiation. This study could have broader implications for how chromosome organization instructs transcription in other cellular contexts and throughout human development.
Reference: Static and Dynamic DNA Loops form AP-1-Bound Activation Hubs during Macrophage Development. Phanstiel, DH, Van Bortle, K, Spacek, D, Hess, GT, Shamim, MS, Machol, I, Love, MI, Lieberman Aiden, E, Bassik, MC, Snyder, MP. Molecular Cell. 2017 September 21. 67(6): 1037-1048.
The 4D Nucleome Project
 The 4D Nucleome Program aims to develop and apply approaches to map the structure and dynamics of the human and mouse genomes in space and time with the goal of understanding how the nucleus is organized and functions. The program will develop and benchmark robust experimental and computational approaches for measuring genome conformation and nuclear organization, and illuminate how they contribute to gene regulation and other genome functions. These efforts will lead to new insights into how the genome is organized, maintained, expressed and replicated, in both normal and disease states.
The 4D Nucleome Program aims to develop and apply approaches to map the structure and dynamics of the human and mouse genomes in space and time with the goal of understanding how the nucleus is organized and functions. The program will develop and benchmark robust experimental and computational approaches for measuring genome conformation and nuclear organization, and illuminate how they contribute to gene regulation and other genome functions. These efforts will lead to new insights into how the genome is organized, maintained, expressed and replicated, in both normal and disease states.
Reference: The 4D Nucleome Project. Dekker J, Belmont A, Guttman M, Leshyk V, Lis J, Lomvardas S, Mirny L, O’Shea C, Park P, Ren B, Ritland Politz C, Shendure J, Zhong S, & the 4D Nucleome
Network. Nature. 2017 September 14.
Visualizing the Nucleus in 3D
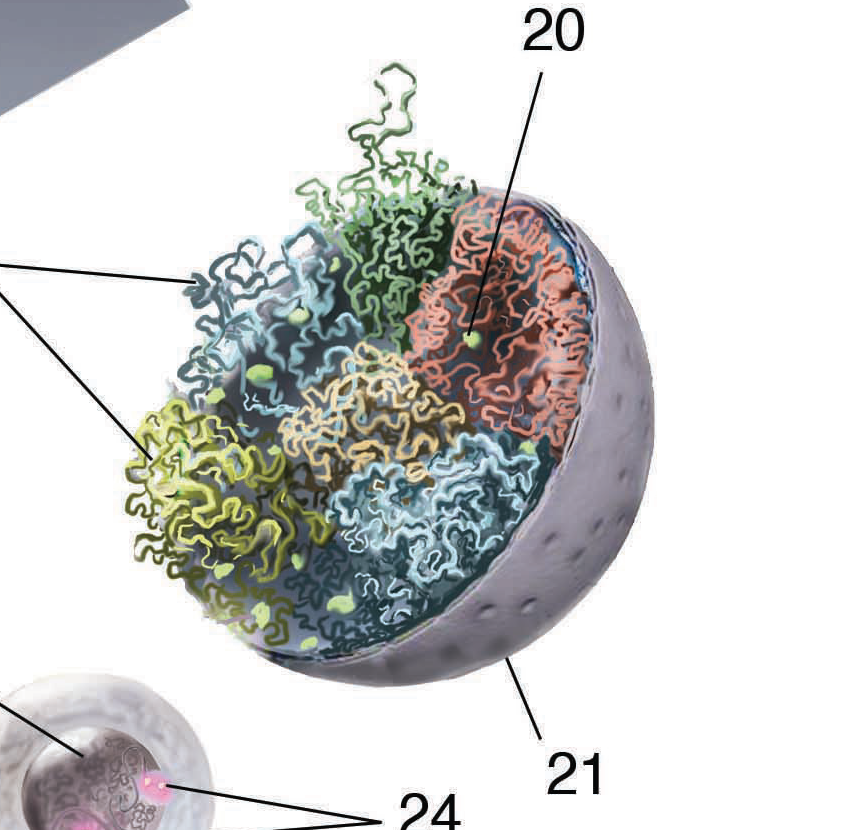
 How DNA is packaged in the nucleus determines how it is used and how genes are expressed. DNA is condensed and packaged as chromatin (a complex of DNA and proteins called histones), which constantly changes as genes are expressed. Understanding chromatin packaging may reveal the structural code for how genes are turned on or off in human health and disease. For example, understanding chromatin packaging could be used to make cancer cells with abnormally structured chromatin “remember” how to be healthier through repackaging chromatin. Toward understanding chromatin packaging in the nucleus, 4D Nucleome (4DN) program grantee Dr. Clodagh O’Shea collaborated with fellow 4DN grantee Dr. Mark Ellisman to develop a new approach to visualize chromatin in 3D space. This method, called ChromET, combines electron microscopy tomography (EMT) and a labeling method that enhances the visualization of DNA in human cell lines. An electron microscope uses a beam of electrons to create an image of a sample and is capable of seeing much smaller objects than a traditional light microscope. The 4DN researchers used ChromET to show that chromatin is flexibly disordered and packed together at different concentrations in the nucleus. This is different from the textbook model of rigid higher-order chromatin folding. This new model of diverse chromatin structures – able to bend at various lengths and achieve different packing concentrations – is important because it provides an explanation for how different parts of the genome could be fine-tuned to make different structures, at different times, with different functions. This research brings us one step closer to discovering how the structural code of our genomes could be used to advance medical care.
How DNA is packaged in the nucleus determines how it is used and how genes are expressed. DNA is condensed and packaged as chromatin (a complex of DNA and proteins called histones), which constantly changes as genes are expressed. Understanding chromatin packaging may reveal the structural code for how genes are turned on or off in human health and disease. For example, understanding chromatin packaging could be used to make cancer cells with abnormally structured chromatin “remember” how to be healthier through repackaging chromatin. Toward understanding chromatin packaging in the nucleus, 4D Nucleome (4DN) program grantee Dr. Clodagh O’Shea collaborated with fellow 4DN grantee Dr. Mark Ellisman to develop a new approach to visualize chromatin in 3D space. This method, called ChromET, combines electron microscopy tomography (EMT) and a labeling method that enhances the visualization of DNA in human cell lines. An electron microscope uses a beam of electrons to create an image of a sample and is capable of seeing much smaller objects than a traditional light microscope. The 4DN researchers used ChromET to show that chromatin is flexibly disordered and packed together at different concentrations in the nucleus. This is different from the textbook model of rigid higher-order chromatin folding. This new model of diverse chromatin structures – able to bend at various lengths and achieve different packing concentrations – is important because it provides an explanation for how different parts of the genome could be fine-tuned to make different structures, at different times, with different functions. This research brings us one step closer to discovering how the structural code of our genomes could be used to advance medical care.
Reference: ChromEMT: Visualizing 3D chromatin structure and compaction of the human genome in interphase and mitotic cells. Ou, HD, Phan S, Deerinck TJ, Thor A, Ellisman, MH, O’Shea CC. Science. 2017 July 28.
In the News: Salk Institute Press Release, NIH Press Release, Study: DNA Folding Patterns Revealed The Scientist
Genes on the Move
 By studying cell-to-cell variability in dividing cells, a research team led by 4D Nucleome (4DN) grantees Drs. Peter Fraser and Amos Tanay revealed that genes don't have a fixed location in the cell, but are constantly moving. The cell cycle is a series of events during which a cell grows, replicates its chromosomes, and then divides. Chromosome conformation capture is a technique used to detect physical interactions between different segments of chromosomes. It can be used to investigate the 3D folding of chromosomes, and to identify where genes on those chromosomes are positioned in the nucleus. Most chromosome conformation capture experiments are done using millions of cells in different phases of the cell cycle, all mixed together. In this study, researchers used chromosome conformation capture and statistical analysis to look at the 3D folding of chromosomes in thousands of individual mouse embryonic stem cells, all separated from one another. Surprisingly, they found that genes don’t have a fixed location in the cell nucleus – rather, genes are continuously moving and change their positions as they progress through different stages of the cell cycle. This is important because changes in gene location can result in changes in gene activity, which can influence human health and disease. Studying chromosomal organization could illuminate how changes in the location of a gene can affect normal development as well as various diseases.
By studying cell-to-cell variability in dividing cells, a research team led by 4D Nucleome (4DN) grantees Drs. Peter Fraser and Amos Tanay revealed that genes don't have a fixed location in the cell, but are constantly moving. The cell cycle is a series of events during which a cell grows, replicates its chromosomes, and then divides. Chromosome conformation capture is a technique used to detect physical interactions between different segments of chromosomes. It can be used to investigate the 3D folding of chromosomes, and to identify where genes on those chromosomes are positioned in the nucleus. Most chromosome conformation capture experiments are done using millions of cells in different phases of the cell cycle, all mixed together. In this study, researchers used chromosome conformation capture and statistical analysis to look at the 3D folding of chromosomes in thousands of individual mouse embryonic stem cells, all separated from one another. Surprisingly, they found that genes don’t have a fixed location in the cell nucleus – rather, genes are continuously moving and change their positions as they progress through different stages of the cell cycle. This is important because changes in gene location can result in changes in gene activity, which can influence human health and disease. Studying chromosomal organization could illuminate how changes in the location of a gene can affect normal development as well as various diseases.
Reference: Cell-cycle dynamics of chromosomal organization at single-cell resolution. Nagano T, Lubling Y, Várnai C, Dudley C, Leung W, Baran Y, Mendelson Cohen N, Wingett S, Fraser P, Tanay A. Nature. 2017 July 6. 547(7661):61-67.
In the News: Cell cycle: Continuous chromatin changes, Nature. Music of the Genes Rises from the Cell Cycle, Genetic Engineering & Biotechnology News.
Genome Organization: Unpacked
 4D Nucleome grantees have combined imaging and genomic methods to discover new insights about certain proteins that impact gene expression and nuclear organization. Chromatin is a substance found within our chromosomes that consists of DNA and protein. The function of chromatin is to efficiently package over 6 feet of DNA into a remarkably small volume – the nucleus of a cell – and protect the DNA structure and sequence. Formation of chromatin structures and nuclear organization are not random, it turns out that chromatin is organized in chromosomal “neighborhoods” within the nucleus. Packaging of DNA into chromatin in these neighborhoods can control gene expression and DNA replication. Yet, the principles behind genome organization in the nucleus are not well understood. Understanding nuclear architecture is important because abnormalities in genome organization are associated with diseases, including cancer and premature aging. One way to study genome organization is through chromosome conformation capture, a technique that is used to detect physical interactions between different segments of the genome. Another method is fluorescent in situ hybridization (FISH), which researchers use to visualize and map the cell’s genetic material through imaging. Although both provide a wealth of information, bringing these approaches together (correlating imaging technologies with genomics and sequencing) will allow researchers to sequence information that they see.
4D Nucleome grantees have combined imaging and genomic methods to discover new insights about certain proteins that impact gene expression and nuclear organization. Chromatin is a substance found within our chromosomes that consists of DNA and protein. The function of chromatin is to efficiently package over 6 feet of DNA into a remarkably small volume – the nucleus of a cell – and protect the DNA structure and sequence. Formation of chromatin structures and nuclear organization are not random, it turns out that chromatin is organized in chromosomal “neighborhoods” within the nucleus. Packaging of DNA into chromatin in these neighborhoods can control gene expression and DNA replication. Yet, the principles behind genome organization in the nucleus are not well understood. Understanding nuclear architecture is important because abnormalities in genome organization are associated with diseases, including cancer and premature aging. One way to study genome organization is through chromosome conformation capture, a technique that is used to detect physical interactions between different segments of the genome. Another method is fluorescent in situ hybridization (FISH), which researchers use to visualize and map the cell’s genetic material through imaging. Although both provide a wealth of information, bringing these approaches together (correlating imaging technologies with genomics and sequencing) will allow researchers to sequence information that they see.
Toward this aim, 4D Nucleome grantees Drs. Arjun Raj, Jennifer E. Phillips-Cremins, and Gerd A. Blobel have used FISH imaging and chromosome capture genomic methods to discover new insights about bromodomain and extraterminal motif (BET) proteins, which help regulate gene expression by controlling chromatin and nuclear organization. Although there are several different types of BET proteins, the role of each type and how they interact with chromatin is not well understood. The study of BET proteins is important because BET inhibitors are a class of drugs with anti-cancer, immunosuppressive, and other effects in clinical trials. In this study, they found that a certain type of BET protein contributes to the formation of boundaries, which help chromatin form chromosomal neighborhoods. This raises the possibility that BET inhibitors can influence gene expression by disrupting boundary function. Using imaging and genomic sequencing methods to gain a clearer understanding of BET proteins will help researchers understand how BET inhibitors work, bringing us one step closer to treating disease.
Reference: The BET Protein BRD2 Cooperates with CTCF to Enforce Transcriptional and Architectural Boundaries. Hsu SC, Gilgenast TG, Bartman CR, Edwards CR, Stonestrom AJ, Huang P, Emerson DJ, Evans P, Werner MT, Keller CA, Giardine B, Hardison RC, Raj A, Phillips-Cremins JE, Blobel GA. Molecular Cell. 2017 April 6. 66(1):102-116.
Studying the Mystery of Genome Organization in Development
 Researchers from the Common Fund’s 4DN Program have developed a new technology to peer inside the cell at the earliest stages of development, revealing important differences in how the DNA from the mother and the father are organized and potentially providing clues about how the multitude of cell types in an organism can arise from a single cell. During fertilization, maternal and paternal DNA coexist in the zygote, which later develops into an embryo following the instructions encoded in the DNA. Understanding how DNA from both parents is organized in the zygote is important because 3D organization of DNA is one way that a cell can control gene expression. Tight regulation of gene expression in these early developmental stages is key to tissue, organ, and organism development. One way to study genome organization is through chromosome conformation capture (Hi-C), a technique that is used to detect physical interactions between sequences of the genome. However, using this method to study the nuclear organization of a single zygote or embryo is challenging because it usually requires hundreds, thousands, or millions of cells.
Researchers from the Common Fund’s 4DN Program have developed a new technology to peer inside the cell at the earliest stages of development, revealing important differences in how the DNA from the mother and the father are organized and potentially providing clues about how the multitude of cell types in an organism can arise from a single cell. During fertilization, maternal and paternal DNA coexist in the zygote, which later develops into an embryo following the instructions encoded in the DNA. Understanding how DNA from both parents is organized in the zygote is important because 3D organization of DNA is one way that a cell can control gene expression. Tight regulation of gene expression in these early developmental stages is key to tissue, organ, and organism development. One way to study genome organization is through chromosome conformation capture (Hi-C), a technique that is used to detect physical interactions between sequences of the genome. However, using this method to study the nuclear organization of a single zygote or embryo is challenging because it usually requires hundreds, thousands, or millions of cells.
To overcome this challenge, 4D Nucleome grantee Dr. Leonid Mirny, and collaborators, have developed a new form of Hi-C technology to analyze cells at a single cell level - a significant technological advancement in this field. Using this method, they found that nuclear architecture is uniquely reorganized during the ococyte to zygote transition in mice and that maternal and paternal DNA are packaged differently in mouse zygotes. The oocyte to zygote transition refers to a critical period of development, during which fundamental changes in nuclear function take place as the egg and sperm give rise to an embryonic genome. This exciting knowledge of the zygotic “ground state” will contribute to our understanding of how cells give rise to an astounding number of different cell types that make up an organism.
In the News: Mom’s and Dad’s Genes Packed Differently in Fertilized Egg, Genetic Engineering & Biotechnology News. In the beginning there was order, Nature.
Reference: Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. Flyamer IM, Gassler J, Imakaev M, Brandão HB, Ulianov SV, Abdennur N, Razin SV, Mirny LA, Tachibana-Konwalski K. Nature. 2017 April 6. 544(7648):110-114.
Understanding Chromosome Structure and Cancer
DNA is not randomly arranged in the nucleus. Instead, nuclear organization is tightly controlled. For example, insulated neighborhoods are loops of DNA that function to maintain normal expression of genes within and outside of the loop. Defects in nuclear organization and the folding of the human genome have been linked to cancer. In a new study, 4D Nucleome investigator Dr. Job Dekker, Ph.D., Howard Hughes Medical Institute Investigator, University of Massachusetts Medical School, and collaborators investigated the causal relationship between chromosome structure and oncogene activation. Oncogenes are genes that have the potential to cause cancer, when activated through dysregulation of gene expression. Using DNA sequences from tumors and targeted mutations in cancer cell lines, they show that disruption of insulated neighborhoods can activate oncogenes. This suggests that disruption of chromatin architecture is causally linked to the formation of tumors. This work represents a step toward understanding 3D chromosome structure and the authors conclude that “understanding these regulatory processes may provide new approaches to therapeutics that have on impact an aberrant chromosome structure”.
Reference: Activation of proto-oncogenes by disruption of chromosome neighborhoods. Hnisz D, Weintraub AS, Day DS, Valton AL, Bak RO, Li CH, Goldmann J, Lajoie BR, Fan ZP, Sigova AA, Reddy J, Borges-Rivera D, Lee TI, Jaenisch R, Porteus MH, Dekker J, Young RA. Science. (6280) 1454 – 8.
A Guiding Light to Study Protein Assembly in Living Cells
 4D Nucleome grantee Dr. Clifford Brangwynne and collaborators have developed a new tool that uses light to manipulate matter inside living cells. Called optoDroplet, this tool helps explain the physics and chemistry behind how cells assemble a mysterious structure called a membraneless organelle. An organelle is a specialized part of a cell having some specific function. For example, the nucleus is an organelle that holds most of the cell’s genetic information. Organelles like the nucleus are walled off from the rest of the cell by a membrane. The cell also uses membraneless organelles that resemble liquid droplets and exhibit dynamic behavior, such as rapid assembly and disassembly of protein building blocks that make up the organelle. When these mechanisms go awry, aggregates of the protein building blocks can form. Protein aggregation is associated with a number of diseases, including amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease) and Alzheimer's disease. Understanding the process by which proteins condense into these droplet-like, membraneless organelles may be used to develop of interventions and treatments for diseases connected with protein aggregation. To better understand this process, Dr. Brangwynne’s group developed optoDroplet. This new tool relies on optogenetics, which involves proteins whose behavior can be altered by exposure to light. Using mouse and human cells, researchers showed that they could create membraneless organelles by switching on the light-activated proteins. They were also able to use this tool to generate protein aggregates, similar to those found in some diseases. The optoDroplet system will help researchers understand the basic mechanisms that underlie self-assembly of membraneless organelles in healthy living cells and may reveal how cells become diseased when this process goes awry.
4D Nucleome grantee Dr. Clifford Brangwynne and collaborators have developed a new tool that uses light to manipulate matter inside living cells. Called optoDroplet, this tool helps explain the physics and chemistry behind how cells assemble a mysterious structure called a membraneless organelle. An organelle is a specialized part of a cell having some specific function. For example, the nucleus is an organelle that holds most of the cell’s genetic information. Organelles like the nucleus are walled off from the rest of the cell by a membrane. The cell also uses membraneless organelles that resemble liquid droplets and exhibit dynamic behavior, such as rapid assembly and disassembly of protein building blocks that make up the organelle. When these mechanisms go awry, aggregates of the protein building blocks can form. Protein aggregation is associated with a number of diseases, including amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease) and Alzheimer's disease. Understanding the process by which proteins condense into these droplet-like, membraneless organelles may be used to develop of interventions and treatments for diseases connected with protein aggregation. To better understand this process, Dr. Brangwynne’s group developed optoDroplet. This new tool relies on optogenetics, which involves proteins whose behavior can be altered by exposure to light. Using mouse and human cells, researchers showed that they could create membraneless organelles by switching on the light-activated proteins. They were also able to use this tool to generate protein aggregates, similar to those found in some diseases. The optoDroplet system will help researchers understand the basic mechanisms that underlie self-assembly of membraneless organelles in healthy living cells and may reveal how cells become diseased when this process goes awry.
In the news: New tool shines a light on protein condensation in living cells, Light-Operated Tool Will Offer New Insights Into Protein Assembly in ALS
Reference: Spatiotemporal Control of Intracellular Phase Transitions Using Light-Activated optoDroplets. Shin Y, Berry J, Pannucci N, Haataja MP, Toettcher JE, Brangwynne CP. Cell. 2017, January 12.
Study Reveals Elaborate X-inactivated Chromosome Structure
 X-inactivation is the process by which one of the two X chromosomes present in female mammals is inactivated. This prevents them from having twice as many X chromosomes gene products as males, who only have a single X chromosome. A Barr body is the structure inside of the nucleus that consists of the inactive X chromosome. Although the Barr body appears to be a condensed blob under a microscope, a new study from 4D Nucleome grantee Job Dekker, Ph.D. and collaborators reveals a highly elaborate structure. Using a variety of methods, including chromosome conformation capture technologies and mouse models, they found that the inactive X chromosome is actually composed of two distinct lobes of inactive DNA. They also found that these lobes were separated by highly repetitive segments of DNA called “microsatellite repeats”. When these microsatellite repeats were removed, the bi-lobed chromosome structure vanished. In another finding of this study, a limited number of active genes in these lobes were separated by topologically associated domains (TADs), which are regions of the genome where DNA interactions frequently occur. This finding is significant because it suggests that TADs may organize gene expression in the inactive X chromosome, at least in neural progenitor cells.
X-inactivation is the process by which one of the two X chromosomes present in female mammals is inactivated. This prevents them from having twice as many X chromosomes gene products as males, who only have a single X chromosome. A Barr body is the structure inside of the nucleus that consists of the inactive X chromosome. Although the Barr body appears to be a condensed blob under a microscope, a new study from 4D Nucleome grantee Job Dekker, Ph.D. and collaborators reveals a highly elaborate structure. Using a variety of methods, including chromosome conformation capture technologies and mouse models, they found that the inactive X chromosome is actually composed of two distinct lobes of inactive DNA. They also found that these lobes were separated by highly repetitive segments of DNA called “microsatellite repeats”. When these microsatellite repeats were removed, the bi-lobed chromosome structure vanished. In another finding of this study, a limited number of active genes in these lobes were separated by topologically associated domains (TADs), which are regions of the genome where DNA interactions frequently occur. This finding is significant because it suggests that TADs may organize gene expression in the inactive X chromosome, at least in neural progenitor cells.
“This is the most detailed molecular view we’ve been able to obtain of the DNA inside the Barr body,” said Dekker. “Under a microscope, the inactive X chromosome is very different than other chromosomes; it looks like a condensed, undefined, inactive ‘blob.’ Our study, using a range of experimental approaches including imaging and genomic methods, describes something else entirely: a highly organized and elaborate structure, rich in features that may silence or activate genes all along the chromosome.”
In the news: Read the press release from the University of Massachusetts Medical School here.
Reference: Structural organization of the inactive X chromosome in the mouse. Giorgetti L, Lajoie BR, Carter AC, Attia M, Zhan Y, Xu J, Chen CJ, Kaplan N, Chang HY, Heard E, Dekker J. Nature. 2016 July 28.
Bursting onto the Transcription Scene
 Transcription occurs when a particular segment of DNA is expressed into RNA. This process appears to take place in intermittent bursts and has been observed in organisms ranging from bacteria to humans. Transcriptional bursting is a term that describes this highly variable occurrence. Enhancers are short regions of DNA that can significantly increase the likelihood that a gene will be transcribed. There are hundreds of thousands of enhancers in the human genome, many of which precisely regulate patterns of gene expression that are required for the differentiation and growth of cells and tissues. To better understand the relationship between transcriptional bursts and enhancers, Dr. Michael Levine, a 4D Nucleome grantee, used quantitative analysis and live-imaging methods in Drosophila embryos in real time. Using this model organism, they report that enhancers regulate the frequency of transcriptional bursts and that strong enhancers produce more bursts than weak ones. They also report that shared enhancers can drive coordinated bursting of two different reporter genes, suggesting the importance of chromosome architecture in control of gene expression.
Transcription occurs when a particular segment of DNA is expressed into RNA. This process appears to take place in intermittent bursts and has been observed in organisms ranging from bacteria to humans. Transcriptional bursting is a term that describes this highly variable occurrence. Enhancers are short regions of DNA that can significantly increase the likelihood that a gene will be transcribed. There are hundreds of thousands of enhancers in the human genome, many of which precisely regulate patterns of gene expression that are required for the differentiation and growth of cells and tissues. To better understand the relationship between transcriptional bursts and enhancers, Dr. Michael Levine, a 4D Nucleome grantee, used quantitative analysis and live-imaging methods in Drosophila embryos in real time. Using this model organism, they report that enhancers regulate the frequency of transcriptional bursts and that strong enhancers produce more bursts than weak ones. They also report that shared enhancers can drive coordinated bursting of two different reporter genes, suggesting the importance of chromosome architecture in control of gene expression.
In the news: Dynamic enhancer–promoter interactions for transcriptional bursting.
Reference: Enhancer Controls of Transcriptional Bursting. Fukaya T, Lim B, Levine M. Cell. 2016 June 9.
Keeping Chromosomes in the Loop

Chromosomes are compacted over 100-fold to form highly condensed structures during cell division, yet the active process of chromosome compaction into loops is not well understood. 4D Nucleome grantee Dr. Leonid Mirny, a professor of physics in MIT’s Institute for Medical Engineering and Sciences, and collaborators have developed model that explains how chromosomes condense into these compact structures. Using computer simulations of chromosomes, the team investigated whether loops could produce the compact chromosomes seen in dividing cells. Their work, reported in three papers published in Cell Reports, eLife, and Biophysical Journal, suggests that chromosome organization relies on proteins that act as molecular motors that pull strands of DNA into increasingly larger loops. The authors report that proteins thought to hold DNA together, cohesion and condensin, can also transform loosely tangled chromosomes into a series of small loops that condense each chromosome. This allows compacted chromosomes to remain segregated from one another. A similar model explains how chromosomes are organized when cells are not dividing, suggesting that this loop extrusion model helps to control which genes are expressed. “Nobody has ever directly observed this mechanism of loop extrusion. If it exists, it will solve lots of problems,” says Dr. Leonid Mirny. “We will know how chromosomes condense, how they segregate, how genes talk to enhancers. Lots of things can be solved by this mechanism.”
References:
Compaction and segregation of sister chromatids via active loop extrusion. Goloborodko A, Imakaev MV, Marko JF, Mirny L. Elife. 2016 May 18.
Chromosome Compaction by Active Loop Extrusion. Goloborodko A, Marko JF, Mirny LA. Biophysical Journal. 2016 May 24.
Formation of Chromosomal Domains by Loop Extrusion. Fudenberg G, Imakaev M, Lu C, Goloborodko A, Abdennur N, Mirny LA. Cell Reports. 2016 May 31.
CRISPRainbow: Observing the Genome in Living Color
4D Nucleome investigators Drs. Thoru Pederson and David Grunwald, and colleagues, have developed a new technology called CRISPRainbow. While most researchers use CRISPR for editing genomes, Pederson, Grunwald and colleagues used CRISPR/Cas9 technology to tag specific locations of the genome, label these locations with fluorescent proteins, and track them in real time under a microscope. Existing technologies are only capable of following three genomic locations at a time in living cells, but CRISPRainbow allows researchers to tag and track up to seven different genomic locations in live cells. "Computers cooperating with spectral filters in the microscope read out combinations of colors and display them as a color that you request," explains Thoru Pederson, Ph.D. "For example, red and green can be yellow. Using the three primary colors and this approach, which is called computational coloring, we can generate an additional three colors.” A seventh label, white, is accomplished by combining all three primary colors. Fluorescent labels such as these are important for studying chromosome dynamics and movements of the genome, which may have important biological consequences.
Learn more about this study from the University of Massachusetts Medical School.
In the news: Read about this study in Genetic Engineering & Biotechnology News.
Reference: Multiplexed labeling of genomic loci with dCas9 and engineered sgRNAs using CRISPRainbow. Ma H, Tu LC, Naseri A, Huisman M, Zhang S, Grunwald D, Pederson T. Nature Biotechnology. 2016 April 18.


