Computational modeling, using computers to simulate the behavior of complex systems through mathematics, physics and/or computer science, is increasingly important to therapeutic development. It allows for the exploration of possibilities in a shorter period of time than studies focused on humans or animals or even isolated cells. Several SPARC awardees are making strides in this area; here we highlight two that are working to improve the understanding of how current and future therapies interact with the heart.
Dr. Colleen E. Clancy’s team used computational modeling to look for structural nooks and crannies where novel heart medications and anesthetic drugs might interact with the heart’s tiny electrical “gates”, or channels. These channels control how and which charged particles enter heart cells and can be sites of action, or “targets” for therapeutics. The model shows how drug molecules might interact in 4D with the structures of the heart’s channels (see video simulations in movie 1 & movie 2 ). This innovative approach essentially ‘digitally screened’ the drugs, revealing it requires two drug molecules to properly bind to the channel as well as identifying additional three-dimensional pathways that drug molecules can take to get to their binding sites. This has important implications for the design of drugs for heart conditions and could help push the field toward smarter designs for new drug therapies before they are tested in animals or humans.
In another SPARC-supported computational modeling effort, Dr. Elenora Grandi’s team studied the importance of a different type of electrical channel in heart function. Working from a recent finding that this channel could be involved in heart disease, they simulated the effects of reducing its activity. In the simulation, this resulted in an increase in the duration of electrophysiological patterns that are associated with potentially dangerous abnormal heart rhythms such as atrial fibrillation. This is additional evidence that the balance of specific electrical activities is critical to healthy heart function. New approaches for managing abnormal heart rhythms could involve maintaining this balance with drugs or devices.
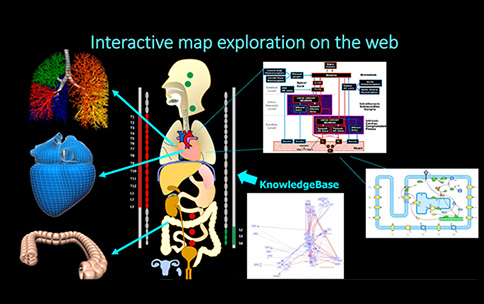
To improve neuromodulation therapies targeting the heart, we will need to understand the specific effects of nerve or spinal cord stimulation on the channel proteins studied by Clancy, Grandi, and others. Each of these studies is impactful in its own right; however, the SPARC program seeks to amplify the impact of this type of research by addressing data and model interoperability. As an example, these two studies both involve heart channels; yet without a shared vocabulary or common framework, the output of one study cannot be used as an input to another without manual curation and reformatting. Such gaps are routine across biomedical science and create delays in making findings useful and translational. Through its Data and Resource Center, the SPARC program is supporting the development of such a common framework, allowing maps and simulations to integrate diverse anatomical and physiological results from many different groups. The resulting resources, whose development you can track at http://sparc.science and MAP-core (a SPARC resource, building interactive visualizations of nerve-organ anatomy and function; see figure), will allow for the generation of new therapeutic hypotheses that leverage a broader multidisciplinary range of biomedical research than in the past.
Reference
- Structural basis for antiarrhythmic drug interactions with the human cardiac sodium channel. Nguyen, P. T., DeMarco, K. R., Vorobyov, I., Clancy, C. E., & & Yarov-Yarovoy, V. . Proceedings of the National Academy of Sciences (2019) 116(8), 2945-2954.
- Transient outward K+ current can strongly modulate action potential duration and initiate alternans in the human atrium. Ni, H., Zhang, H., Grandi, E., Narayan, S. M., & & Giles, W. R. American Journal of Physiology-Heart and Circulatory Physiology (2018) 316(3), H527-H542.
- K+ Channel Isoforms Regulate Human Atrial Arrhythmogenesis. Haibo Ni, Henggui Zhang, Elenora Grandi, Sanjiv M. Narayan, and Wayne R. Giles. AJP-Heart and Circulatory Podcasts.


