Does your heart race when you are exercising and slow down when you are sleeping? Hopefully, it does because precise control of heart rate changes like these can influence medical outcomes, making the neural connections between your brain and heart important targets for devices that modulate the neural pathways running throughout your body (the autonomic nervous system). Historically, the variability in clinical trial results hampered being able to prove the therapeutic efficacy of neuromodulation devices for patients at risk of heart failure. One possible reason is that identifying specific neural connections can be difficult—like finding which wire amongst thousands of wires connects your TV to the cable box. If we improve upon current tools, adding cell-type specificity to existing maps of neural structures and electrical signaling, then we can empower clinicians and device developers to design more specific neuromodulation strategies and decrease treatment variability.
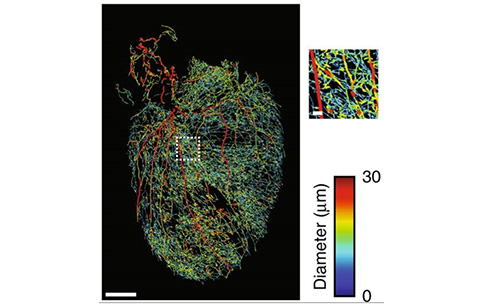
To this end, SPARC awardee Kalyanam Shivkumar and his collaborators are clearing a way for other researchers — literally. Dr. Shivkumar’s team made mouse hearts transparent, a critical step in an innovative multi-stage approach that ultimately allowed them to create 3D visualizations of the neural connections that impact heart rate. After using structure-preserving clearing methods to make mouse hearts easy to see through, they used genetically engineered viruses to “tag” and fluorescently trace specific neurons as a way to build 3D visualizations of the intricate circuits that receive input from nerve structures like the spinal cord and the vagus nerve (image from publication shown). They also showed that similar genetic techniques could be used to make only certain neuron types responsive to light, which could enable more specifically targeted neuromodulation in the future. This is crucial to improving the efficacy of neuromodulation devices because better targets mean new ways to treat heart disease!
Reference
- Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Rajendran, P.S., Challis, R.C., Fowlkes, C.C., Hanna, P., Tompkins, J.D., Jordan, M.C., Hiyari, S., Gabris-Weber, B.A., Greenbaum, A., Chan, K.Y., Deverman, B.E., Münzberg H., Ardell J.L., Salama G., Gradinaru V., and Shivkumar K. Nature communications (2019) 565(7739), 361.


