Partnerships and New Indications - Device Portal
As part of the Translational Partnerships and New Indications component of Phase 1 of the SPARC program, SPARC supported partnerships between industry and SPARC investigators to advance the study of functional neuromodulation. The following table shows a list of companies that signed a Memorandum of Understanding (MOU) with NIH to participate in this program component. The table provides basic information on the devices and support that each company made available at the time for applicants seeking NIH funding.
Please note that these MOUs are not currently active, and this information is maintained to archive SPARC activities.
| Company | Product | Application / Original Indication | Accessories | Device information and company support |
|---|---|---|---|---|
| Blackrock Microsystems | Utah arrays (NeuroPortTM and slanted arrays) | Stimulation/recording for cortical and peripheral applications | Pneumatic electrode inserter system, NeuroPort data acquisition system |
|
| Boston Scientific | PrecisionTM stimulation systems | Spinal cord stimulation | Various percutaneous and surgical paddle stimulation leads | Device Information |
| VerciseTM stimulation systems | Deep brain stimulation | DBS Guide software, DBS standard or directional leads | ||
| CorTec | Brain Interchange® and AirRay® Electrode | Implantable system for chronic open and closed loop interaction | Hermetic Encapsulation technology |
|
| CVRx | BAROSTIM NEOTM
| Baroreflex activation therapy for resistant hypertension and heart failure | Electrical stimulation and data acquisition hardware and software |
|
| Medtronic | Activa RC+S | General purpose research system (similar in capability to Activa RC/PC used in DBS) | Nexus (-D and -E) Algorithm Development Toolkit, Vectris MRI leads, Reveal LINQ Cardiac Monitor |
|
| Enterra II | Gastric electrical stimulation | |||
| InterStim II | Sacral neuromodulation | |||
| RestoreSensor | Spinal cord neuromodulation | |||
| Micro-Leads | Various electrode technologies | General purpose neural interface technology | Conformal arrays, STIM-GRID high density arrays, Precision electrodes, Multi-contact cuffs |
|
| NeuroNexus | Conventional and high-definition thin-film electrodes | General purpose neural interface technology | External neural recording and stimulation systems |
|
| Ripple | LinkTM and Link-STM
| Stimulation/recording for neurophysiology and neuroprostheses research | Grapevine Neural Interface System, 3D printing of flexible electrodes, software support |
|
| Stimwave | Freedom Stimulator System | Peripheral nerve or spinal cord wireless neurostimulators for pain management | External transmitter for power |
|
Application Process –Steps used for applying to SPARC Phase 1 New Market Indications funding opportunities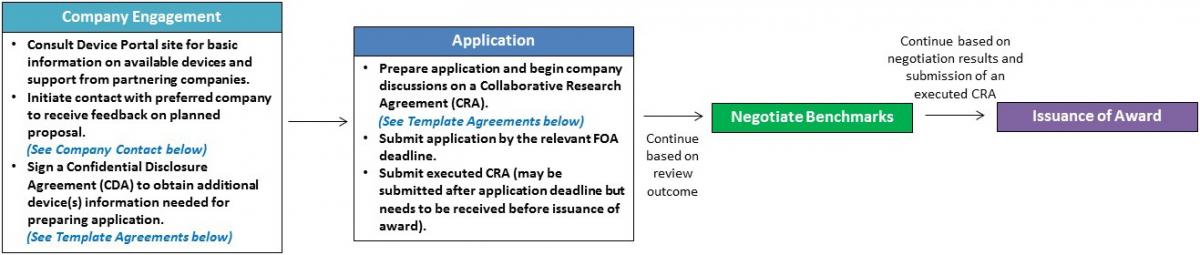
Template Agreements
The following template documents may be used to streamline creating partnerships between investigators and device companies. Investigators are strongly encouraged to consult early with their institutional technology transfer or sponsored research office in order to confirm that the terms and conditions of the CDA and CRA for the selected device are at or near acceptable to their institution.
CDA - Confidential Disclosure Agreement
This template document can be signed by academic researchers to allow confidential discussions of proprietary details with a given company regarding the capabilities of a device prior to submitting SPARC funding announcement applications. In some cases the template language may be used “as is” whereas in other cases it may be a starting point for discussions.
CRA - Collaborative Research Agreement
This is a template document to be used for agreements between device manufacturers and academic research institutions to form partnerships for submission of SPARC funding announcement applications. The goal of this document is to provide standardized terms covering essential components of such agreements (e.g., intellectual property, data and publications, reporting requirements, etc.). In some cases the template language may be used “as is” whereas in other cases it may be a starting point for discussions.
MOU - Memorandum of Understanding
The purpose of this document is to describe the nature of the signatory company's agreement to participate in the program. The document goes over the process under which Collaborative Research Agreements will be reached with academic researchers wishing to use the company's device(s) prior to receiving NIH funding through the SPARC program. Each signatory company has provided a description of the materials and support it is willing to make available for this purpose and has agreed to use the template Collaborative Research Agreement for partnering with research institutions for this program.


