Science Highlights
The Interdisciplinary Research program has transitioned from Common Fund support. For more information, please visit http://commonfund.nih.gov/Interdisciplinary.
 This is your brain on stress
This is your brain on stress

Acute and chronic stress can take a high toll on both physical and mental health. Drs. Amy Arnsten, Carolyn Mazure, and Rajita Sinha, part of the Interdisciplinary Research Consortium on Stress, Self-Control, and Addiction at Yale University, have published an article in the April 2012 issue of Scientific American that describes how your brain reacts to stress, and how that can lead to the all too familiar feeling of “freezing” when faced with an important task, like giving a speech or taking a test. In times of stress, the brain’s “executive control center,” the prefrontal cortex, essentially shuts down, allowing more primitive brain regions that are responsible for compulsive behaviors and emotional responses to take over.
REFERENCES
Arnsten A, Mazure CM, and Sinha R. Everyday stress can shut down the brain’s chief command center. Scientific American, April 2012, 48-53.
- Read the Scientific American article...
- Read more about the Interdisciplinary Research Consortium on Stress, Self-Control, and Addiction...
- Read more about the Interdisciplinary Research program...
 Obese people may have more difficulty resisting high calorie foods
Obese people may have more difficulty resisting high calorie foods

Dr. Rajita Sinha and colleagues at Yale University, part of the Interdisciplinary Research program’s Interdisciplinary Research Consortium on Stress, Self-Control, and Addiction, have discovered that blood sugar levels can affect parts of the brain that regulate the desire for high calorie foods. Their results, published in The Journal of Clinical Investigation, show that when blood sugar levels are low, like when a person is hungry, parts of the brain related to motivation and reward are active and the desire for high calorie foods increases. When blood sugar levels are normal, the motivation and reward centers quiet down while brain regions associated with logical reasoning and impulse control become active, and the desire for high calorie foods decreases. The most surprising finding, however, was that obese individuals show increased brain activity in the reward and motivation centers when blood sugar is low, but lack activity in the impulse control centers when blood sugar is normal. This brain activation pattern suggests that in obesity, cravings for high calorie foods are harder to resist even if the person is not hungry. These results may provide an important clue in the understanding of the biological basis of obesity, and may lead to the development of more effective ways to control eating behavior and weight gain.
REFERENCES
Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, Constable RT, Sherwin RS, and Sinha R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. The Journal of Clinical Investigation 2011; 121(10): 4161-9. PMID: 21926468.
- Read more about the Interdisciplinary Research program…
- Read more about the Interdisciplinary Research Consortium on Stress, Self-Control, and Addiction…
 Interdisciplinary consortium trainee collaboration uses technique developed in teeth to study the heart
Interdisciplinary consortium trainee collaboration uses technique developed in teeth to study the heart

What do teeth and the heart have in common? At first, you might think the answer is, “Not much.” But two trainees in the Interdisciplinary Consortium SysCODE (Systems-based Consortium for Organ Design and Engineering) have demonstrated that a novel technique used to analyze hard-to-detect proteins in tooth development can also be used to study heart valve maintenance and health. Drs. Peggi Angel and David Nusinow were both working in the SysCODE Consortium on separate projects when they met for the first time at the SysCODE biannual meeting. At the meeting, Dr. Nusinow presented a novel method for protein detection in tooth development. Dr. Angel realized that although the mechanical and chemical cues needed to make a tooth are quite different than what is needed to maintain a heart valve, methodologies developed to analyze the development of one organ part could, in theory, be applied to understanding the other.
Working together, these two scientists published a paper identifying key components of the “scaffold” of protein components outside of cells, called the extracellular matrix (ECM), that are critical for heart valve function. The researchers found several proteins that had not previously been associated with heart valve function, and gained novel insights into distinct mechanisms that regulate pulmonary versus aortic valve functions. Importantly, these observations may explain why valve function is frequently not preserved in patients when pulmonary valves are used to correct aortic valve disease. In addition, another analysis method correlated the proteins identified with processes involved in valvular malformations.
Though this collaboration could have happened outside of an Interdisciplinary Research Consortium, the Consortium allowed Drs. Angel and Nusinow the opportunity to interact before Dr. Nusinow’s method was published, hastening the pace of their research. Therefore, in addition to the scientific value of this publication by two interdisciplinary trainees, the collaboration exemplifies how interdisciplinary research at the Consortium can accelerate the advancement of biomedical research.
References:
Angel PM, Nusinow D, Brown CB, Violette K, Barnett JV, Zhang B, Baldwin HS, and Caprioli RM. Networked-based characterization of extracellular matrix proteins from adult mouse pulmonary and aortic valves. Journal of Proteome Research 2011, 10, 812-823. PMID: 21133377.
 Hitting the Target: Molecule Hones in on Cancer Cells
Hitting the Target: Molecule Hones in on Cancer Cells
A major obstacle in the fight against cancer is finding treatments that target and kill only the cancerous cells without damaging healthy cells. Drs. Todd Golub and Stuart Schreiber, supported in part by the Common Fund’s Interdisciplinary Research Consortium for Genomic Based Drug Discovery, along with their colleagues, have discovered a molecule that can selectively kill cancer cells but does not harm normal cells. As reported in the July 14 issue of Nature, this cancer-fighting molecule is piperlongumine, a natural product derived from the plant Piper longum (long pepper). Piperlongumine inhibited tumor growth in mice that were injected with human bladder, breast, lung, or melanoma cancer cells, as well as in mice genetically engineered to develop breast cancer, and it did so more effectively than the chemotherapy drug Taxol (paclitaxel). In both cell cultures and in mice, piperlongumine had no detectable toxic effects on healthy cells, even at extremely high concentrations. The researchers found that piperlongumine works by targeting a cellular process that differs between cancer cells and normal cells. Cancer cells have a much higher rate of metabolism than normal cells, and consequently they have increased levels of toxic reactive oxygen species (ROS). To tolerate high levels of ROS, cancer cells rely heavily on several different anti-oxidative enzymes that protect against these harmful molecules. Piperlongumine diminishes anti-oxidative enzyme activity, causing ROS levels in cancer cells to increase beyond the threshold for cell death. Normal cells, which have slower metabolic rates and lower levels of ROS, are not as dependent on these anti-oxidative enzymes and so are not harmed by piperlongumine. These exciting results demonstrate a novel strategy for the treatment of cancer by targeting a previously unexplored cellular pathway, and may pave the way for future development of effective cancer drugs with limited side effects.
REFERENCES
Raj L, Ide T, Gurkar AU, Foley M, Shenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, and Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011; 475(7355):231-4. PMID: 21753854.
 Fountain of Youth? Worms Reveal New Clues About Long Life
Fountain of Youth? Worms Reveal New Clues About Long Life

Severely reducing calorie intake has been shown to extend lifespan in yeast, worms, and flies, and to delay age-related diseases in some mammals; however, the mechanisms underlying this phenomenon are largely unknown. Scientists in the Common Fund’s Interdisciplinary Research Consortium for Geroscience at the Buck Institute for Age Research have identified a class of molecules called N-acylethanolamines (NAEs) that alert worms when calorie intake is low, and trigger responses that slow aging and increase lifespan. Surprisingly, these molecules are endocannabinoids, which are naturally occurring molecules in animals that are related to the active ingredient in marijuana (Cannibis sativa). Published in the May 12th issue of Nature, the study shows that when worms are fed a low-calorie diet, their levels of NAEs decrease. If the worms are genetically modified or treated with chemicals to reduce their levels of NAEs, they live longer even if they eat normal amounts of food. The opposite effect is also true: increasing the levels of NAEs in these worms, even if they are on a restricted diet, negates the effect of dietary restriction on increasing lifespan. The results suggest that NAEs may signal when nutrient levels are low, and trigger a biological response that slows aging and increases lifespan. Further research is needed to determine whether similar effects will be observed in mammals, including humans.
REFERENCES
Lucanic M, Held JM, Vantipalli MC, Klang IM, Graham JB, Gibson BW, Lithgow GJ, and Gill MS. N-acylethanolamine signaling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature, May 12, 2011. 473: 226-229.
De Petrocellis L and Di Marzo V. Why fasting worms age slowly. Nature, May 12, 2011. 473: 161-163.
 Mouse Genetics Leads to New Clues for Human Psychiatric Disorders
Mouse Genetics Leads to New Clues for Human Psychiatric Disorders

Several psychiatric disorders, including attention deficit hyperactivity disorder (ADHD), drug and alcohol addiction, and schizophrenia, are characterized by poor impulse control and difficulty inhibiting certain behaviors. These traits are referred to as behavioral inflexibility, which is thought to be partially under genetic control. However, the genes responsible have been difficult to identify in humans. In a paper available online March 10, 2011 in the journal Biological Psychiatry, researchers in the Common Fund’s Interdisciplinary Research program’s Consortium for Neuropsychiatric Phenomics report that they have identified several genes associated with behavioral inflexibility in mice, and that these findings might be applicable to humans as well. To identify genes that underlie behavioral inflexibility, Dr. David Jentsch and colleagues from the University of California Los Angeles and the University of Tennessee first tested 51 genetically different strains of mice for the ability to reverse their behavior in a learned task. To successfully complete this task, mice had to learn to poke their nose into an opening either on the left or right side of the cage in order to receive a food reward. Once the mice mastered this skill, they had to unlearn which side to poke their nose into, and re-learn to poke their nose on the opposite side. The number of tries it takes a mouse to reverse its behavior indicates how much behavioral flexibility and impulse control the mouse has. The researchers reasoned that by looking at both the genes and behaviors of the mice, they could find genetic differences that were associated with the behavioral differences. Indeed, the researchers zeroed in on a region of the mouse chromosome 10 that contains several genes that influence behavioral flexibility. One gene, Syn3, regulates chemical communication in the brain and has been inconclusively linked to schizophrenia in humans. Another gene, Nt5dc3, is a gene of unknown function that has been associated with ADHD. The current research suggests that both of these genes should be investigated further to discover what role they may play in human psychiatric disorders, and also demonstrates a new way to use mouse behavior and genetics to find genes that may contribute to complex behaviors in humans.
Reference:
Laughlin RE, Grant TL, Williams RW, Jentsch JD. Genetic dissectioin of behavioral flexibility: reversal learning in mice. Biological Psychiatry, 2011 June 1; 69(11): 1109-16. Epub 2011 March 9. PMID: 21392734.
 Major Finding in Interdisciplinary Trainee Led Project Reveals How One Molecule- Adiponectin- Exerts Broad Protective Effects in Different Disease States
Major Finding in Interdisciplinary Trainee Led Project Reveals How One Molecule- Adiponectin- Exerts Broad Protective Effects in Different Disease States

Internal Medicine Postdoctoral Fellow, Dr. William Holland, led a study published in the January edition of Nature Medicine that sheds new light on the mechanism by which adiponectin- a molecule that plays a critical role in metabolism and obesity- protects against damage in the context of different disease states like diabetes, obesity, and cardiac cell death. Adiponectin can do many things from promoting insulin sensitivity in the liver to preventing cell death in the pancreas and heart. The study shows that it exerts its effects by regulating a lipid known as ceramide. Adiponectin binds to a receiving protein-or “receptor”- on the surface of cells in the target organ such as the liver and this binding activates the receptor in such a way that it cleaves/cuts ceramide molecules. The resulting reduced amount of ceramide in the body is protective because this lipid is associated with insulin resistance, cell death, and inflammation. In addition, another type of lipid called sphingosine is released from the cleaved ceramide and this lipid gets a phosphate group added to it. The so-called “sphingosine-1-phosphate” has the opposite effect of ceramide. So, in effect, adiponectin is able to turn a harmful lipid in to a beneficial one that increases insulin sensitivity and reduces cell death and inflammation.
Reference:
Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nature Medicine, 2011 Jan; 17(1): 55-63. Epub 2010 Dec 26. PMID: 21186369.
 Colorful solution to extending lifespan
Colorful solution to extending lifespan
Researchers at the Common Fund’s Interdisciplinary Research Consortium for Geroscience have discovered that the laboratory dye Thioflavin T (ThT) extends median lifespan in roundworms by 60%. ThT- otherwise known as “Basic Yellow No. 1”- is a common dye used to stain clumps of proteins comprising amyloid plaques found in the brains of Alzheimer’s disease patients. The current research suggests that ThT may extend lifespan in worms by activating a type of stress response in cells, and in this heightened state of alert, cells increase their monitoring and control of proteins, suppressing their aggregation.
Reference:
Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature, 2011 April 14; 472(7342): 226-9. Epub 2011 Mar 30. PMID: 21451522.
 Scientists Identify Brain Cells Responsible For Anti-Diabetic Actions of Serotonin
Scientists Identify Brain Cells Responsible For Anti-Diabetic Actions of Serotonin
Serotonin is an important brain chemical, or neurotransmitter, that is well-studied for its effects on mood, emotion, sleep, and energy regulation. Serotonin activity in the brain is known to exert anti-diabetic effects by maintaining blood glucose levels, but until now, it was unclear which brain cells (neurons) regulate these effects. Dr. Joel Elmquist and colleagues at the Taskforce for Obesity Research at Southwestern (TORS) Consortium, one of nine interdisciplinary research consortia funded by the Common Fund, have recently identified the group of neurons that mediate the anti-diabetic actions of serotonin. In the December 2010 issue of Nature Neuroscience, Dr. Elmquist and colleagues demonstrate that serotonin signaling in POMC neurons of the hypothalamic region of the brain regulates glucose homeostasis and insulin sensitivity. To study the effects of serotonin signaling in POMC neurons, researchers compared two groups of genetically engineered mice. In one group of mice, serotonin signaling was eliminated in every cell in the body. In the second group of mice, serotonin signaling was eliminated in every cell except for POMC neurons. When comparing these two groups of mice, researchers discovered that mice with no serotonin signaling anywhere developed metabolic problems such as insulin resistance in the liver, while mice with serotonin signaling in POMC neurons alone did not have these metabolic problems. These results point to POMC neurons as a critical mediator of serotonin’s anti-diabetic actions. A better understanding of how blood glucose homeostasis and insulin sensitivity are regulated by serotonin may offer insights into how to prevent the metabolic imbalances sometimes seen in patients who are taking anti-depressant or anti-psychotic drugs that affect serotonin levels.
REFERENCES
Xu Y, Berglund ED, Sohn JW, Holland WL, Chuang JC, Fukuda M, Rossi J, Williams KW, Jones JE, Zigman JM, Lowell BB, Scherer PE, Elmquist JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat Neurosci 2010 Dec; 13(12): 1457-9. PMID: 21037584.
 Regulation of Proteins Plays an Important Role in Aging
Regulation of Proteins Plays an Important Role in Aging
Dr. Gordon Lithgow and colleagues at the Buck Institute’s Geroscience Consortium, one of nine interdisciplinary research consortia funded by the Common Fund, have discovered that the translation of key proteins may underlie the ability of long-lived mutant worms to survive extreme heat conditions that are fatal to normal worms. Translation is a cellular process that makes proteins from a type of genetic material known as RNA, and by altering this process, long-lived worms may be protected from environmental stresses like heat. This ability to withstand environmental stress is thought to be related to the worms’ increased longevity, and thus discoveries about how these long-lived worms withstand stress may also be helpful for understanding the process of aging.
Why study worms?
The biological study of aging is a complex and interdisciplinary field, with researchers studying how environmental stresses, genetic changes, and diet can impact lifespan. Worms, such as the commonly used laboratory model C. elegans, are a valuable research model for aging studies because they have a short lifespan (typically two to three weeks) that can easily be observed in the laboratory. Additionally, many factors that increase lifespan in worms, such as dietary restriction, also appear to improve health and increase lifespan in monkeys and people. There are many different types of mutant worms that are resistant to environmental stress and have increased lifespan. The long-lived mutant worms in this study are genetically modified so they are less responsive to insulin, a hormone that regulates metabolism. It is well-known that decreases in insulin responsiveness result in longer lifespan in worms, but the exact reason why is unclear.
New ideas about aging
Inside each cell, genes encoded by DNA are first copied into RNA in a process known as transcription. After transcription, RNA is translated into proteins. Many previous studies on long-lived worms had attributed their ability to withstand environmental stresses on changes in transcription. While transcription undoubtedly plays an important role in protection from stress and increased longevity, this study demonstrates that translation of key proteins is critical for surviving extreme heat and contributes to increased lifespan in long-lived worms. This discovery suggests a novel pathway through which insulin affects lifespan by regulation of key proteins in the cell. A better understanding of insulin responsiveness, protein regulation, and protection from environmental stress may lead to new insights on how to increase longevity and promote healthy aging.
REFERENCES:
McColl G, Rogers AN, Alavez S, Hubbard AE, Melov S, Link CD, Bush AI, Kapahi P, Lithgow GJ. Insulin-like signaling determines survival during stress via posttranscriptional mechanisms in C. elegans. Cell Metab. 2010 Sep 8;12(3):260-72. PMID: 20816092
 Deciphering Fragile X-Associated Diseases
Deciphering Fragile X-Associated Diseases

Fragile X-associated tremor/ataxia syndrome (FXTAS) is a late onset neurodegenerative disorder mainly affecting men 50 to 80 years old. The syndrome is associated with a host of problems including tremors, imbalance, numbness in the feet, and memory loss including dementia.
FXTAS is caused by a genetic alteration in the Fragile X mental retardation (FMR1) gene whereby a portion of the gene containing the DNA trinucleotide “CGG” gets repeated in a region of the gene known to control gene activity -- the 5’ untranslated region. While most people have between 6 and 45 CGG repeats in their FMR1 gene, people with FXTAS have between 55 to 200 CGG repeats in their FMR1 gene. Having extra CGG repeats is thought to produce five to ten times more defective FMR1 RNA than usual. The defective RNA cannot be used make the FMR1 protein needed for neurons to develop normally. Instead, the defective protein accumulates in the neurons where it can be toxic to them.
Interestingly, people who have greater than 200 CGG repeats in their FMR1 gene have a related condition called Fragile X syndrome that occurs at birth as opposed to later in life. Fragile X syndrome is associated with mental retardation, attention deficit hyperactivity disorder (ADHD) and severe anxiety, and genetically predisposes an individual to the development of autism. In Fragile X syndrome, the FMR1 gene is “silenced” because the extra CGG repeats change the gene’s structure so much that the cell cannot produce RNA from the gene. This, in turn, disrupts the regulation of other genes involved in neurodevelopment. The number of CGG repeats in the FMR1 gene actually tends to increase with each generation such that there are grandfathers with FXTAS that have grandsons with Fragile X syndrome.
Dr. Paul Hagerman and colleagues at the Neurotherapeutics Research Institute at the University of California-Davis, one of nine Interdisciplinary Research Consortia funded by the Common Fund, are exploring whether FXTAS is a late onset neurodegenerative stage of an early onset neurodevelopmental disorder. That is, is it possible that neurons in people with FXTAS are already damaged by birth as they are in people with Fragile X, since both related disorders involve increased numbers of CGG nucleotide repeats in the same FMR1 gene?
To test their hypothesis, they studied neurons in mouse models of FXTAS that mimic the human disease. In one-day old mice, they found that neurons from the hippocampal region of the brain that functions in memory already looked and functioned abnormally, and by 12 to 24 weeks of age, the mice showed progressive defects in spatial processing. The findings suggest that FXTAS is an end stage disorder that results from a cellular impairment that is present at birth and persists throughout life, highlighting the opportunity to develop early biological, chemical and behavioral interventions.
REFERENCES
Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R and Pessah1 IN. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet 2010 19(1):196-208. PMID: 19846466.
Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. Progressive spatial processing deficits in a mouse model of the fragile X permutation. Behav Neurosci. 2009 Vol 123(6) 1315-1324. PMID: 20001115.
Adams PE, Adams JS, Nguyen DV, Hessl D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, DeCarli C, Hagerman PJ, R.J. Hagerman RJ. Psychological Symptoms Correlate With Reduced Hippocampal Volume in Fragile X Premutation Carriers. 2009. Am J Med Genet Part B. PMID: 19908235.
 Novel Approaches to Restore Fertility in Cancer Patients
Novel Approaches to Restore Fertility in Cancer Patients
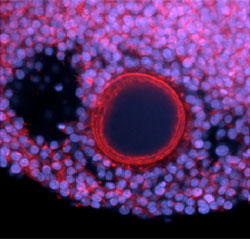
Human follicle grown in the lab. The oocyte and surrounding somatic cells are health and developing and behaving similar to follicles in vivo. Original work was published in: Xu, Barrett et al. (2009) In vitro grown human ovarian follicles from cancer patients support oocyte growth. Human Reproduction, 24:2531. Image was captured on a LSM 510 Zeiss confocal at Northwestern University’s Cell Imaging Facility.
Effective cancer therapies offer patients longer survival times. Unfortunately, common therapies such as chemotherapy are toxic to immature eggs that are housed in round structures in the ovary known as follicles. Girls and young women who receive life-saving cancer therapies may diminish or lose their ability to conceive children in the future. Infertility in female cancer patients may also reduce their quality of life. Doctors can offer few options to treat their infertility using current methods. For example, pre-pubescent girls are not mature enough to undergo ovarian hormone stimulation to collect eggs that can be frozen for later use, and the procedure is dangerous for older women with estrogen-positive breast cancer. New approaches are needed to restore fertility in female cancer survivors.
The Common Fund’s Interdisciplinary Research program supports an Oncofertility Consortium that is developing new technologies to restore fertility in female cancer patients who may become infertile because of treatment. The investigators have engineered a novel, three-dimensional cell culture system that allows human follicles containing immature eggs to grow into mature follicles and eggs that could then be used in fertility treatments. Girls and young women can donate ovarian tissue containing the immature follicles prior to beginning cancer treatment. The follicles can be grown into mature eggs in vitro and used to restore fertility when the time is right to conceive.
Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum. Reprod. 2009 Oct;24(10):2531-40. Epub 2009 Jul 13. Link: http://humrep.oxfordjournals.org/cgi/content/full/24/10/2531



