More than half of hearing loss present at birth is inherited through specific genetic changes that are passed down from parents. Many genetic conditions, including genetic deafness, have no approved treatment. This is why the NIH Common Fund funded the Somatic Cell Genome Editing (SCGE) program to help reduce the burden of diseases and conditions caused by genetic changes and to accelerate the translation of genome editing approaches into the clinic.
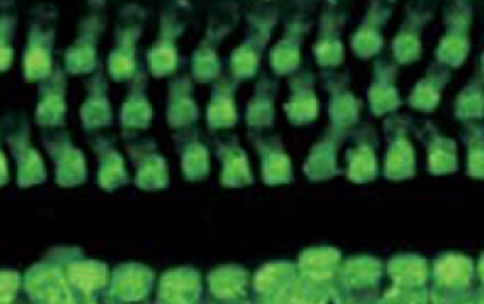
In a study published in Science Translational Medicine, SCGE researcher Dr. Zheng-Yi Chen and his colleagues used gene editing to restore hearing in adult mice with a rare form of genetic deafness called autosomal dominant deafness-50 (DFNA50). DFNA50 is caused by mutations in the microRNA-96 (MIR96) gene. MicroRNAs (miRNAs) are a family of molecules that help cells control the kinds and amounts of proteins they make. MiRNAs play a crucial role in the development of the inner ear and are required for normal hearing. Mutations in MIR96 are linked to abnormal hair cells and the reduction in hair cell numbers in early development in mice. Hair cells act as sensors to detect sound and motion and are crucial for hearing. Targeting hair cells offers an opportunity for genetic intervention in patients with DFNA50.
The researchers delivered a gene editing tool called CRISPR/Cas9 into the hair cells of the inner ear of mice with the MIR96 mutation. They used an adeno-associated virus (AAV) that can be engineered to deliver gene editing tools into target cells. They tested the therapy on newborn mice before the onset of hearing loss and in adult mice with hearing loss. The therapy was effective in improving long-term hearing in both stages of hearing loss, and earlier intervention proved more beneficial. The researchers also showed evidence that the intervention was safe and precise. The delivery virus did not insert itself into the genome of the hair cells and editing was not detected in unintended tissues. A similar approach can be applied to target more than one MIR96 mutation, offering a promising way to treat multiple forms of hearing loss caused by different mutations in the same gene.
Dr. Chen and his colleagues demonstrated the viability of a gene editing therapy that offers an opportunity for genetic intervention in patients with DFNA50 and other forms of hearing loss. For the application in humans, the therapy requires further preclinical studies in other animal models to improve the safety and efficacy.
Reference: Zhu W, Du W, Rameshbabu AP, Armstrong AM, Silver S, Kim Y, Wei W, Shu Y, Liu X, Lewis MA, Steel KP, Chen ZY. Targeted genome editing restores auditory function in adult mice with progressive hearing loss caused by a human microRNA mutation. Science Translation Medicine. 2024 July 10. DOI:10.1126/scitranslmed.adn0689.


