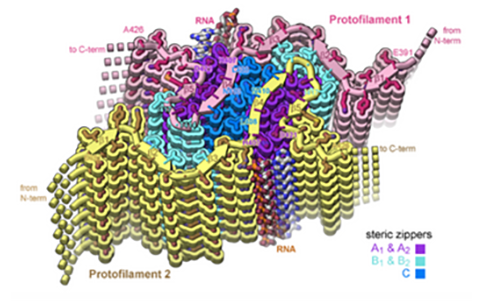
In neurodegenerative diseases including Alzheimer’s and Amyotrophic Lateral Sclerosis (ALS), proteins form abnormal clumps in the brain that can contribute to neuron loss. Among these proteins, Tau is an important protein for normal physiologic function, but is also well known to form rope-like aggregates, or clumps, known as tau fibrils in neurodegenerative diseases. Evidence suggests that RNA binds to tau proteins and contributes to the development of these tau fibril structures, but little is known about the structure of these complexes.
With the help of the Stanford-SLAC CryoEM Center (S2C2), one of three Common Fund-supported National Centers for Cryoelectron Microscopy (CryoEM), a team of researchers led by David S. Eisenberg, Ph.D., at the University of California, Los Angeles, determined the structure of full-length tau fibrils bound to RNA. According to the authors, this structure could represent an early step in formation of tau fibrils and may offer useful information about how development of tau-RNA fibrils might be reversed.
To study the relationship between tau fibrils and RNA, researchers formed tau fibrils with RNA from mice and viewed their structure using cryoEM, a method that provides detailed images of biological molecules without the use of structure-altering dyes or fixatives necessary in other methods. The structure revealed strands of RNA molecules running up and down along the sides of the long tau fibrils. When they added enzymes to break up the RNA, the tau fibrils dissolved, revealing that the RNA was necessary for protein integrity.
According to the researchers, the cryoEM structure, which is nearly detailed enough to see the individual atoms of tau RNA fibrils, provides a structural explanation of how tau-RNA fibrils could be reversed. If this structure found using cryoEM samples also forms within the brains of human patients, it could be a step toward designing structure-based therapeutics to break up tau fibrils that lead to neurodegeneration.
The project used resources from the NIH Common Fund’s Transformative High-Resolution CryoEM program, which is broadening access to CryoEM for biomedical researchers by creating national service centers and cultivating a skilled workforce through CryoEM training materials, providing detailed images of molecules that could not be obtained using previous methods such as X-ray crystallography.
Reference
- Abskharon R, Sawaya MR, Boyer DR, Cao Q, Nguyen BA, Cascio D, Eisenberg DS. Cryo-EM structure of RNA-induced tau fibrils reveals a small C-terminal core that may nucleate fibril formation. Proc Natl Acad Sci U S A. 2022 Apr 12; 119 (15):e2119952119. doi: 10.1073/pnas.2119952119. PMID: 35377792


