2012 Awardees
Asa Abeliovich, Ph.D.
Columbia University Health Sciences, NY
Project Title: Generation and Integration of New CNS Neurons by In Vivo Directed Conversion
Grant ID: R01-NS-081701
The proposed research will pursue improving cell and animal models for human brain disorders, as well as develop novel cell therapy strategies for brain diseases. Recently, our group succeeded in the directed conversion of human patient skin fibroblasts to neurons, achieved by introducing a 'cocktail' of pro-neural factors. Such cell reprogramming approaches may allow for the facile generation of replacement cells for some brain disorders. The ultimate goal is to apply this technology to model Alzheimer disease pathology, with the intention of pursuing disease mechanisms and test potential therapies.

David B. Allison, Ph.D.
University of Alabama at Birmingham, AL
Project Title: Energetics, Disparities, & Lifespan: A Unified Hypothesis
Grant ID: R01-AG-043972
The evolution and ontogeny of lifespan at the species and individual level, the energetics of the organism in its environment, the storage of metabolizable energy as body fat, and socioeconomic disparities within populations all seem intricately related and yet the nature of these interrelations is poorly understood. Indeed questions as fundamental as why people age remain open and subsidiary questions such as why caloric restriction leads to increased lifespan and why lower socioeconomic status is related to obesity in developed countries also remain unanswered. The research proposes a unified model informed by evolutionary thinking about life strategies which connects these phenomena. In this model, aging or more precisely senescence is not something that passively happens to people as the result of environmental insults or from metabolizing fuel. That is, mortality rate or the rate of aging is seen as (partially) internally regulable phenomenon much like the control of body temperature in homeotherms in which the regulated rate is responsive to perceptions about the energetic state of the environment. From this perspective, it is perceptions of the energetic security of the environment that are a key factor in linking these phenomena. We test this hypothesis using model organisms followed by mechanistic work involving internal clock regulation and other potential mechanisms.
David Altshuler, M.D., Ph.D. and Chad A. Cowan, Ph.D.
Broad Institute, Inc., MA / Harvard University
Project Title: Isogenic Human Pluripotent Stem Cell-Based Models of Human Disease Mutations
Grant ID: R01-DK-097768
Development of new and more effective therapies for Type 2 diabetes (T2DM) and coronary artery disease (CAD) requires improved understanding of disease mechanisms. Genome sequencing in human populations identifies genes and mutations underlying the inherited contribution to disease susceptibility, but progress is slowed by two central challenges: (a) determining which DNA changes are functional, and which are benign, and (b) developing cellular assays that faithfully recapitulate the function and molecular pathology of disease genes and variants thereby identified. Our project addresses these challenges by integrating data and methods from human genetics, genome engineering, and stem cell biology, and is made possible by three recent advances: (a) next-generation genome sequencing identifying candidate disease mutations in human clinical populations, (b) development of methods to rapidly and precisely edit any given genome sequence of interest in human pluripotent stem cells (hPSCs) using engineered TAL effector nucleases (TALENs), and (c) protocols to differentiate hPSCs into cell populations with characteristics of hepatocytes, adipocytes, and pancreatic beta cells. By engineering human stem cells to carry specific mutations, and by differentiating these engineered stem cells into physiologically relevant human metabolic cell types, we will make it possible to study the impact of large numbers of gene variants on human cell biology and function. By relating the functions of gene mutations and cell biological processes with the phenotypes of human patients, we will provide pathophysiological insights and practical in vitro assays to guide development of therapeutics for these challenging diseases, which remain among the leading causes of morbidity and mortality in the United States and around the world.
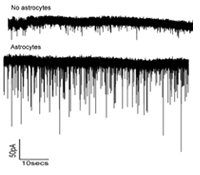
Ben A. Barres, M.D., Ph.D.
Stanford University, CA
Project Title: An Astrocytic Basis for Humanity
Grant ID: R01-NS-081703
Astrocytes are a major cell type in the brain long thought to be passive support cells, but our recent studies have shown that astrocytes powerfully control the formation and function synapses in the brain. In this research we will test the hypothesis that the superior cognitive abilities of humans compared to rodents is, at least in part, the result of an evolutionary increase in the ability of human astrocytes to control synapse formation and function compared to rodent astrocytes.
Helen M. Blau, Ph.D.
Stanford University, CA
Project Title: Telomere Extension Using Nucleoside-Modified mRNA and Exosomes as a Novel Therape
Grant ID: R01-AR-063963
Telomeres comprise DNA sequences at the ends of chromosomes which shorten by about 20-100 base pairs per year on average in humans due to oxidation and incomplete DNA replication during the cell cycle. When telomeres become critically short, chromosomes form chromosome-chromosome fusions which can lead to cancer, and the exposed chromosome ends are recognized as double-stranded breaks that activate DNA damage repair responses that lead to cell death or senescence and consequent tissue and organ dysfunction. Long telomeres protect the ends of chromosomes, and in our laboratory we have shown that short telomeres make the difference between a fatal and non-fatal muscular dystrophy. People with short telomeres are also at greater risk of heart disease, cancer, vascular dementia, Alzheimer’s, and other diseases. Thus, there is a strong motivation to develop methods to safely extend telomeres. We propose to overcome current limitations in telomere extension with two high-impact and broadly-applicable tools: a transient therapeutic designed to extend telomeres safely, rapidly, and reliably, and a delivery vehicle that will allow our novel therapeutic to be administered to a broad range of cells and tissues. We will demonstrate the efficacy of these tools in cells from human Duchenne Muscular Dystrophy patients. Our ultimate goal is to develop telomere extension as a therapeutic tool to help prevent, delay, or treat the many diseases in which short telomeres play a major role.
Emery N. Brown, M.D., Ph.D.; Edward S. Boyden, M.D., Ph.D.; Ken Solt, M.D.; Matthew A. Wilson, Ph.D.
Massachusetts institute of Technology, MA / Massachusetts General Hospital
Project Title: Redesigning General Anesthesia
Grant ID: R01-GM-104948
In the United States, more than 100,000 patients receive general anesthesia daily to safely undergo most surgical and many non-surgical procedures. Use of anesthetic drugs by non-anesthesiologists in intensive care units and outpatient settings continues to grow. At the same time, anesthesia-related morbidity, including intra-operative awareness, altered neurological development and delirium in children and cognitive dysfunction in the elderly remain significant problems. Despite the central role of anesthesiology in modern healthcare, research in this field is overly focused on deciphering the anesthetic and toxic mechanisms of current drugs with no attention to developing new approaches. We propose to redesign general anesthesia by combining optogenetic, electrical and pharmacological manipulations in rodent models to create this behavioral- physiological state through precisely timed control of specific brain circuits. If successful this research will provide a new fundamental understanding of brain arousal control, and eventually, new anesthesiology practices including: neurophysiologically- designed approaches to creating general anesthesia; reduction in morbidity; improved brain function monitoring; safer anesthesia care by non-anesthesiologists; and possibly novel therapies for arousal disorders such as depression, insomnia, pain and coma.
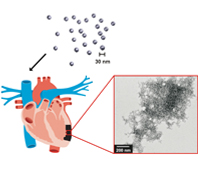
Karen L. Christman, Ph.D. and Nathan C. Gianneschi, Ph.D.
University of California San Diego, CA
Project Title: Autonomously Assembling Nanomaterial Scaffolds for Treating Myocardial Infarction
Grant ID: R01-HL-117326
Each year approximately three quarters of a million Americans will have a new myocardial infarction (MI), and approximately half a million will have a recurrent MI. No current therapies exist that directly address the negative left ventricular remodeling process that occurs post-MI and results in heart failure. There is therefore a strong clinical need to develop novel therapies to treat MI. This research program will establish a new paradigm in the design of treatments for healing heart tissue post-MI. Specifically our plan is to develop self-assembling materials programmed to form a healing scaffold in damaged heart tissue immediately following MI. In the proposed, unprecedented approach, the materials will be designed to be injected intravenously rather than directly into heart tissue, and will target and self-assemble in the MI, providing a scaffold to recruit endogenous cells for cardiac repair.
Robert B. Darnell, M.D., Ph.D.
Rockefeller University, NY
Project Title: Mapping the Mechanisms of Protein Synthesis-Dependent Synaptic Plasticity
Grant ID: R01-NS-081706
This research proposes to change the current approach to understanding the molecular basis of memory. Our approach challenges the current focus on quantitative identification of synaptic RNAs by developing new technologies to address protein synthesis-dependent synaptic plasticity: single-synapse-CLIP and synaptic translational profiling. These will allow us to redefine the problem from two new superimposed perspectives: the need to identify regulated RNA-protein complexes in specific synapses, and the need to define their role in translational regulation. Specific synapses will be studied: the apical dendrites of cerebellar Purkinje neurons (a site of motor learning), of CA1 pyramidal neurons in the stratum moleculare of the hippocampus (a site of associative memory), and of layer V pyramidal neurons of the visual cortex (a site of activity- dependent plasticity). Key RNA-protein complexes known to be present in the dendrite and to bind 3’ UTRs and/or be involved in translational regulation will be studied. These complexes will be compared with a delineation of all ribosome-mRNA synaptic complexes present in the same dendrites, allowing us to validate interactions by identifying translationally regulated synaptic mRNAs (synaptic translational profiling). Regulated dendritic RNAs will be further validated by assessing for their translational state in well-studied paradigms of protein synthesis-dependent synaptic plasticity. These studies offer the possibility of transforming our view of the nature and regulation of local synaptic mRNAs that underlie memory, setting the stage for new insight into neurologic diseases of memory such as Alzheimer’s and other neurodegenerative diseases.
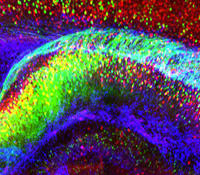
Karl Deisseroth, M.D., Ph.D.
Stanford University, CA
Project Title: CLARITY: Fully-Assembled Biology
Grant ID: R01-MH-099647
Psychiatric disease represents the leading cause of disability both in the U.S. and worldwide but is poorly understood, in part because complex brain function depends upon small-scale cellular structures interacting via long-range circuitry— two very different perspectives which are difficult for researchers to link, and to maintain under investigation simultaneously. In general, achieving high-resolution information on any biological system typically comes at the expense of the global perspective that is often essential to understanding. This research project will directly address this challenge, in part by developing and applying new chemical engineering-based technologies to rapidly transform intact, impermeable and opaque biological tissue into macromolecule-permeable (for labeling and interfacing) and transparent form. The CLARITY technology will be developed with compatible readout methods for extracting volumetric activity and tissue history, information which can then be linked to global wiring and local molecular phenotypes obtained from the very same tissue or organism. The approach will be developed in the vertebrate central nervous system, challenging for its low accessibility and high complexity, but CLARITY is applicable to any biological system including to the human brain and to models of neurological and psychiatric disease.

Robert W. Gereau, Ph.D.; Michael R. Bruchas, Ph.D.; John Rogers, Ph.D.
Washington University, MO / University of Illinois, Urbana-Champaign
Project Title: Multimodal Biocompatible MicroLED Devices for Diverse Neuroscience Applications
Grant ID: R01-NS-081707
Recently, an elegant technique was developed allowing for unprecedented control and specificity in mapping the molecular and cellular properties of neural circuits. This technique, termed “optogenetics,” takes advantage of light-sensitive ion channels, pumps, and G-protein coupled receptors to control neuronal activity and signaling with exceptional temporal precision. Some of the most burning questions in the neurobiology of pain, affect and addiction lend themselves particularly well to the implementation of optogenetic neural modulation techniques, yet these still require drastic miniaturization of circuits, power and light sources for full implementation in diverse neuroscience applications. This project aims to develop biocompatible multimodal microscale inorganic light-emitting devices (µ-ILED) capable of permanent integration with the central and peripheral nervous systems for studying and treating neurological diseases.
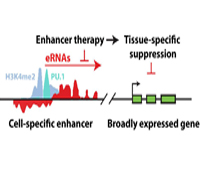
Christopher K. Glass, M.D., Ph.D.
University of California San Diego, CA
Project Title: Enhancer Therapy
Grant ID: R01-CA-173903
Current efforts are to determine the biochemical and biological roles of sequence- specific transcription factors and their associated co-regulators at gene-specific and genome-wide scales. This research project will use a combination of biochemical, cellular and genetic model systems are used, incorporating macrophage-specific knockouts, microarray technologies, massively parallel sequencing and bioinformatics approaches, to unravel the contributions of specific factors to the development of specialized macrophage functions in immunity and the pathogenesis of inflammatory diseases.

Paul Marasco , Ph.D.
Cleveland VA Medical Research/ED/FDN, OH
Project Title: Restoring Upper Limb Movement Sense to Amputees; A Move Towards Natural Control of Prosthetic Limbs
Grant ID: R01-NS-081710
This research seeks to understand the organization and (function/operation) of sensory neural systems in order to develop methods for restoring function to injured populations. One of the primary focus areas of our research is working to integrate physiologically relevant sensory feedback with prosthetic limbs. To this end we employ a variety of approaches that interweave disciplines such as electrophysiology, psychophysics, biomedical engineering and cognition. Our research team is composed of an interconnected and communicative network of clinicians, engineers, and scientists. This helps us to provide pathways from basic science discoveries that can be used to address clinical needs with transition directly to patient care.
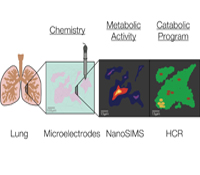
Dianne K. Newman, Ph.D.
California Institute of Technology, CA
Project Title: Geobiological Approaches to Understanding Pulmonary Infections In Situ
Grant ID: R01-HL-117328
Pulmonary infections by microorganisms, such as Mycobacterium tuberculosis and Pseudomonas aeruginosa, are annually responsible for the morbidity and mortality of millions of immunocompromised individuals worldwide. Despite the availability of drugs that successfully eradicate these pathogens in vitro, they are far less successful in vivo. Due to the challenges of working in situ, most studies of infectious disease agents (IDA) are conventionally performed with representative isolates and imperfect disease models in the laboratory; by necessity, they are highly reductionist. Very few direct measurements of the physiological state of drug-tolerant populations in the host exist, and little is known about which metabolic pathways are actually at play, much less how they change over time in response to co-evolving conditions within the lung. We will tackle this critical knowledge gap using an approach inspired by geobiology. Geobiologists are experienced in studying the growth and metabolism of microbial populations in poorly accessible natural habitats by combining molecular biology and stable isotope geochemistry. We propose to study IDA within infected lungs using these tools, with the goal of defining the composition, growth rate and metabolism of the microbial community at different stages in disease progression with high spatial resolution.
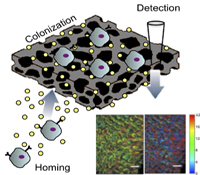
Lonnie D. Shea, Ph.D. and Vadim Backman, Ph.D.
Northwestern University, IL
Project Title: An In Vivo Metastasis Sensor
Grant ID: R01-CA-173745
The onset of distant metastasis marks the stage of cancer progression where the disease is no longer considered curable. Currently, clinicians are unable to determine when metastases have occurred until the cells have colonized one or more distal sites and often affected the function of the effected organ. We propose an early detection system based on developing an implant that would recruit metastatic cancer cells and a sensor to identify when these cells have colonized the implant. Recruiting the metastatic cancer cells would initially function to reduce the burden of circulating tumor cells and limit their colonization in other tissues. Upon detection of the metastatic cells at the implant, the implant could be retrieved to analyze the biology of the cells as well as the stromal/immune environment in order to develop patient-specific interventions based on the biology of the metastatic site rather than the primary tumor. Investigating the design of implants that recruit metastatic cancer cells is expected to further define the biology of the pre-metastatic niche. Taken together, the development of an implant to recruit metastatic cells and a non-invasive technology to monitor growth could transform current clinical approaches to cancer treatment.
Sergey S. Shevkoplyas, Ph.D.
Tulane University of Louisiana, LA
Project Title: Eliminating Mediators of Toxicity from Stored Blood
Grant ID: R01-HL-117329
With nearly 15 million units of red blood cells (RBCs) transfused to about 5 million patients in the U.S. every year, RBC transfusion is one of the most commonly prescribed therapies for hospital inpatients. Most transfusions involve RBCs that had been stored in an anticoagulant-preservative solution at 1-6°C for up to 6 weeks. During hypothermic storage, a significant fraction of stored RBCs becomes irreparably damaged and the storage medium accumulates known mediators of toxicity as byproducts of RBC metabolism and degradation. Infusion of these toxic mediators and the irreparably damaged cells into the recipient during transfusion reduces the therapeutic efficacy of transfusion and contributes to multiple adverse outcomes in about 1-2% of U.S. citizens every year. The goal of this project is to develop technology for high-throughput removal of irreparably damaged cells and toxic mediators accumulating in the storage medium from RBC units during the transfusion process.
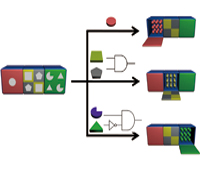
Milan N. Stojanovic, Ph.D. and Hao Yan, Ph.D.
Columbia University Health Sciences, NY / Arizona State University
Project Title: Theranostic Nano-Objects: Basic Principles and Initial Applications
Grant ID: R01-GM-104960
In this project, we will systematically explore the design of smart nanobjects able to transduce molecular level sensing of indicators of health status (a.k.a. biomarkers) into conformational changes coupled to downstream physiological effects. Our results will lay groundwork for broadly applicable families of theranostic (providing therapy upon diagnostics) devices that would be able to autonomously monitor and correct disease states.
Thomas Tuschl, Ph.D.; Uwe Ohler, Ph.D.; Dinshaw Patel, Ph.D.
Rockefeller University, NY / Duke University / Sloan-Kettering Institute for Cancer Research
Project Title: Posttranscriptional Regulation by mRNA-Binding Shuttling and Transport proteins
Grant ID: R01-GM-104962
All mRNA molecules are subject to posttranscriptional gene regulation (PTGR) by hundreds of RNA-binding proteins (RBPs) involving sequence-dependent modulation of splicing, cleavage and polyadenylation, editing, transport, stability, and translation. The major goal of this application is to identify and characterize the interaction network of mRNA-binding transport and shuttling proteins at the sequence, structural, and functional level and to establish theoretical and experimental models that relate these features to RNA transport processes and PTGR. Our approach involves deep sequencing methodologies suitable for broadly mapping interaction sites between RBPs and their RNA targets in human cells, followed by integrated annotation of binding sites on transcripts across libraries via a probabilistic graphical modeling approach to identify prevalent states reflecting particular site configurations. As computed interaction models emerge, these will be studied by biophysical and structural methods using natural, as well as designed RNA recognition element representing RNA ligands together with their respective recombinant proteins. We are placing emphasis on obtaining larger RNP structures to provide insights into “chromatin”-like or less repetitive higher-order packaging of mRNA and assay its possible role during mRNA transport. We anticipate the identification of numerous important regulatory regions in mRNAs, as well as uncover mRNAs and RBPs particularly vulnerable to mutation and deregulation in disease states.
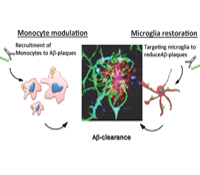
Howard L. Weiner, M.D.
Brigham and Women’s Hospital, MA
Project Title: Role of the Innate Immune System in Aging and Development of Alzheimer's Disease
Grant ID: R01-AG-043975
A major health problem relates to progressive cognitive decline with age and the threat of Alzheimer’s disease (AD). We postulate, based on new data we have obtained, that the brain’s innate immune system plays a central role in successful aging and resistance to AD. The role of the innate immune system in aging and AD has received limited study and is poorly understood. This is due in part to confusion regarding microglial cells and how they relate to the peripheral innate immune system. We discovered unique biomarkers and microRNA/gene signatures for resident microglia in the brain vs. recruited monocytes in both mice and humans. This advance presents a unique opportunity to determine the functions and dysfunctions of innate immune cells in brain aging and the development of AD. We will study the innate immune system both in animal models and aging human subjects and in human postmortem tissue.
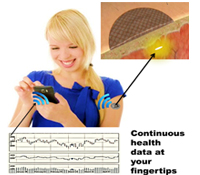
Natalie A. Wisniewski, Ph.D. and Mike McShane, Ph.D.
PROFUSA, Inc., CA / Texas A&M University
Project Title: Implantable Multi-Analyte Sensors for the Continuous Monitoring of Body Chemistries
Grant ID: R01-EB-016414
The existing health care system requires an individual to visit a health care facility to conduct point-in-time tests to monitor even the most basic health status markers, which can miss fluctuations in body chemistries that are vital to accurate diagnoses, particularly in high-risk populations. Continuous multi-chemistry health sensors have the potential to dramatically change health care by paving the way for decentralization of health care delivery and shifting the focus away from reactive treatment to preventative maintenance. We propose to transform current testing paradigms by developing highly miniaturized, injectable, sensors for continuous and simultaneous monitoring of multiple body chemistries. Sensor molecules that glow (somewhat like fireflies) when they come in contact with certain biomarkers are embedded into specially engineered tissue-like biomaterials. The sensor molecules are embedded in soft, tissue-like biomaterials that become part of the tissue in which they are injected, and do not cause the typical foreign body rejection response. The sensors are injected under the skin and monitored optically using a miniaturized, wireless, Band-Aid-like reader for continuous measurement or a hand-held wand for periodic self-measurement, depending on the clinical need. The data may be viewed via cell phone or at a remote location, allowing the individual, physician, or other care providers to access medical data without the need for in-person examination until a critical threshold is met.
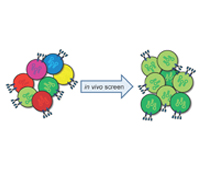
Kai W. Wucherpfennig, M.D., Ph.D. and Stephen Gottschalk, M.D.
Dana-Farber Cancer Institute, MA / Baylor College of Medicine
Project Title: Reprogramming of T cells for the Treatment of Melanoma
Grant ID: R01-CA-173750
The research project will develop a systematic in vivo discovery approach for identification of critical genes and pathways that limit the anti-tumor activity of cytotoxic T cells. Our hypothesis is that shRNAs which target critical inhibitors in dysfunctional T cells can reprogram them to undergo substantial expansion in tumors. T cells will be genetically modified with shRNAs and then transferred into tumor-bearing mice so that enrichment of particular shRNAs within tumors can be quantified. This in vivo approach will also be used to address a second related problem in oncology, the identification of combination therapies that act in a highly synergistic manner on defined cellular pathways. We will use these discoveries to enhance the activity of human T cells that express a ‘chimeric antigen receptor’ (CAR) directed against a molecule on the surface of melanoma cells. The therapeutic activity of these human T cells will be tested in a xenotransplant mouse model on human melanomas, as a key step towards translation of our discoveries into the clinic.



