Science Highlights
2016 Data Now Available in the State Health Practices Database
 The State Health Practices Database for Research (link is external) (SHPDR), which captures cross-sectional and longitudinal information on states’ statutes and regulations, has been recently updated and now contains data from 2016. This data will add to the data from 2010, 2012, and 2014 that were already available and will enable researchers to more effectively perform research on health services outcomes. SHPDR contains information on selected state health regulatory practices affecting population level health outcomes and can be linked with various other existing datasets for hypothesis-driven, cross-cutting, multidisciplinary scientific research.
The State Health Practices Database for Research (link is external) (SHPDR), which captures cross-sectional and longitudinal information on states’ statutes and regulations, has been recently updated and now contains data from 2016. This data will add to the data from 2010, 2012, and 2014 that were already available and will enable researchers to more effectively perform research on health services outcomes. SHPDR contains information on selected state health regulatory practices affecting population level health outcomes and can be linked with various other existing datasets for hypothesis-driven, cross-cutting, multidisciplinary scientific research.
New Mathematical Model Explores Issues Affecting Personalized Medical Care
 Using people’s genetic sequences to personalize medical care shows great promise. This kind of care is already a reality for some cancer treatments, where patients’ genetic sequences guide treatment decisions. As more opportunities to develop personalized interventions are identified, how can we decide which ones offer the greatest benefits in the context of real-world medical care?
Using people’s genetic sequences to personalize medical care shows great promise. This kind of care is already a reality for some cancer treatments, where patients’ genetic sequences guide treatment decisions. As more opportunities to develop personalized interventions are identified, how can we decide which ones offer the greatest benefits in the context of real-world medical care?
Common Fund Health Economics program researchers Drs. Anirban Basu, Josh Carlson, and David Veenstra built on an existing mathematical model of the “Expected Value of Individualized Care,” or EVIC, to explore how real-world issues in medical care could affect the value of personalized medicine approaches. Their findings indicate that changes in several factors affecting the delivery of medical care can have profound effects on the potential value of developing a new personalized treatment. Factors explored in the paper include the rate at which a new medical approach is incorporated in clinical practice over time, insurance coverage and reimbursement rates, the precision of a test to determine who would benefit from the treatment, and how much doctors personalize treatments based on experience and observable patient traits. The rate at which a new technology diffuses through the health care system is particularly important, as it cannot be known at the time technology development decisions are made. The expanded EVIC model can show the extent to which more rapid technological diffusion increases the potential value of a new test. This can be done both from the perspective of a manufacturer and from the perspective of an insurer who considers both costs and clinical benefits.
By accounting for issues like the diffusion of new medical technologies, the expanded EVIC model is an important tool to help us understand how to identify and develop personalized medicine approaches with the greatest potential benefit to those who need them.
Learn more about the research:
This research was supported in part by Cooperative Agreement U01-AG047109 awarded under the Determinants and Consequences of Personalized Health Care and Prevention initiative of the NIH Common Fund’s Health Economics program, for which Dr. Veenstra is Principal Investigator and Drs. Basu and Carlson are co-investigators.
Dr. Basu received the 2016 Award for Excellence in Methodology in Pharmacoeconomics and Health Outcomes Research from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) for this paper. Learn more about ISPOR and the award (link is external).
Reference
A Framework for Prioritizing Research Investments in Precision Medicine. Basu A, Carlson JJ, Veenstra DL. Med Decis Making. 2016 Jul;36(5):567-80. doi: 10.1177/0272989X15610780. Epub 2015 Oct 26. (Note: Full text may require institutional journal access).
Should I Stay, or Should I Go? Lengthy Travel Times May Reduce the Benefit of Specialized Care for Stroke Patients
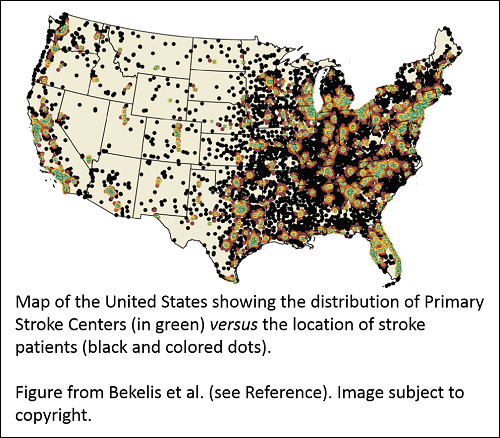 Dartmouth College researchers – including Health Economics program grantee Dr. Jonathon Skinner – compared survival data for over 800,000 elderly stroke patients to street-level geographical data to see if the benefit of admission to a nationally certified Primary Stroke Center (PSC) would outweigh the transit time. They found a 1.8% reduction in deaths 7 and 30 days after a stroke for patients admitted to a PSC where they could receive specialized care. However, if a patient lived more than 90 minutes from the PSC, there was no benefit to admission at the PSC rather than at a closer local hospital.
Dartmouth College researchers – including Health Economics program grantee Dr. Jonathon Skinner – compared survival data for over 800,000 elderly stroke patients to street-level geographical data to see if the benefit of admission to a nationally certified Primary Stroke Center (PSC) would outweigh the transit time. They found a 1.8% reduction in deaths 7 and 30 days after a stroke for patients admitted to a PSC where they could receive specialized care. However, if a patient lived more than 90 minutes from the PSC, there was no benefit to admission at the PSC rather than at a closer local hospital.
The results show transit time is an important consideration for elderly stroke patients. The current geographical distribution of PSCs puts 16.4% of study patients at least 90 minutes away by ground transportation from the nearest PSC. The researchers speculate options like air-lifting patients to PSCs may improve outcomes. Ultimately, further research is needed to identify the best combination of approaches to improve access to centers certified for specialized stroke care and stroke outcomes.
Read a press release (link is external) from Dartmouth College about the study.
Reference
Primary Stroke Center Hospitalization for Elderly Patients with Stroke: Implications for Case Fatality and Travel Times. Kimon Bekelis, MD; Nancy J. Marth, MS, MSN; Kendrew Wong, BS; Weiping Zhou, MS; John D. Birkmeyer, MD; Jonathan Skinner, PhD. JAMA Intern Med. Published online July 25, 2016. doi:10.1001/jamainternmed.2016.3919 (Note: Full text may require institutional journal access).
State Health Practice Database for Research Makes a Splash at Academy Health
NIH and contracting staff presented a poster during the session: “Public and Population Health,” at the Academy Health Research Meeting (link is external) on June 27, 2016. The poster (link is external) describes the State Health Practice Database for Research (SHPDR), a new resource of state-level data on health practices that can be used with statistical software, and linked with existing health and economic data for hypothesis-driven research. The SHPDR poster was specially selected for the June 27 "Poster Walk (link is external)" where leaders in the field gave students and fellows a guided tour of posters demonstrating impactful research. The SHPDR is anticipated to be available to researchers in the fall of 2016.
ASHEcon Pre-Conference Workshop on NIH Funding: June 12, 2016
NIH Health Economics Common Fund Working Group members will be presenting a pre-conference workshop at the 6th Biennial Conference of the American Society for Health Economists (link is external) (ASHEcon) on June 12, 2016 from 1-4:30 PM. The workshop will cover topics such as:
- Writing strong NIH funding applications
- Interpreting the recent guidance on Health Economics priorities at the NIH
- Identifying relevant research priorities of individual NIH Institutes and Centers
- And more!
See the Conference Program (link is external) for location details.
 Public Resource on Diffusion of Medical Technologies
Public Resource on Diffusion of Medical Technologies
Health Economics grantee, Dr. Jonathon Skinner, of the Dartmouth Institute for Health Policy and Clinical Practice, recently unveiled a publicly available resource that describes the diffusion of medical interventions across U.S. geographic regions over time. The online platform (link is external) offers interactive tools to aid in understanding innovation across a spectrum of new and established treatments.
How Public Investment in Long-term Research might Stimulate Private Investments
 Imagine you bought a new house and want to plant trees to shade its lawn. You have several options. You could plant a fast-growing variety that will start to provide shade sooner, or you could plant a hardier, but slower-growing variety that will be slower to produce shade. A lot of factors can affect your decision. How long do you plan to live in the house? Does the city offer incentives to plant the hardier variety? This decision is similar to the one faced by those conducting research and development (R&D) for therapeutics. Do they choose to invest in short-term projects that can be commercialized sooner, or do they invest in long-term projects that potentially have a greater impact for consumers?
Imagine you bought a new house and want to plant trees to shade its lawn. You have several options. You could plant a fast-growing variety that will start to provide shade sooner, or you could plant a hardier, but slower-growing variety that will be slower to produce shade. A lot of factors can affect your decision. How long do you plan to live in the house? Does the city offer incentives to plant the hardier variety? This decision is similar to the one faced by those conducting research and development (R&D) for therapeutics. Do they choose to invest in short-term projects that can be commercialized sooner, or do they invest in long-term projects that potentially have a greater impact for consumers?
NIH Common Fund-supported researcher, Dr. Heidi Williams, and her colleagues, found that private firms tend to invest in short-term R&D projects. In looking at privately-funded R&D in cancer treatments from 1973 to 2011, the researchers found significantly more investment in R&D for late-stage cancer drugs rather than drugs to treat early-stage cancer or for cancer prevention. Late-stage cancer treatments are generally shorter-term investments. They move more quickly through clinical trials partially because cancer trials often measure a drug’s effectiveness in terms of improved survival. Improved survival for late-stage cancer patients may be measured in months, while for early-stage cancer patients, it may be measured in years.
Dr. Williams explored several ways to make long-term R&D investments more attractive. One way might be to change patents to provide longer protections for therapeutics that take longer to get to market. Two other options involve the investment of public funds. Much like a city offering financial incentives to plant a hardy, slow-growing tree, public funds could be used to directly encourage private R&D investments in long-term projects. Dr. Williams also explored the option of using earlier end-points in clinical trials that indicate a drug is safe and effective without waiting for survival data. Public investment to develop earlier clinical end-points in cardiovascular trials almost certainly played an important role in bringing drugs like statins to the market faster. Similar investments in developing earlier clinical end-points for cancer or other clinical trials might pay-off in the long run. This would be much like a city making well-developed saplings of the hardy trees available for purchase, so that you would not have to wait as long for them to grow and produce shade.
Do Firms Underinvest in Long-Term Research? Evidence from Cancer Clinical Trials (link is external). Budish Eric, Benjamin N. Roin, and Heidi Williams. American Economic Review. July 2015. 105(7): 2044-85. Please note that access to the full-text article may require institutional access. Read a brief article about Dr. Williams’ research in the New York Times: “Why Preventing Cancer Is Not the Priority in Drug Development (link is external).”
Read a brief article about Dr. Williams’ research in the New York Times: “Why Preventing Cancer Is Not the Priority in Drug Development (link is external).”
Dr. Williams's paper was selected for the 2016 Arrow Award by the International Health Economics Association. Learn more about the award (link is external).
Dr. Heidi Williams was selected as a MacArthur Foundation fellow for the class of 2015! Read more about Dr. Williams’ research (link is external) or watch a brief video (link is external) about her story from the MacArthur Foundation.
Economics of Prevention Workshop
![]() On August 27 and 28, 2015, the National Institutes of Health (NIH) Health Economics Common Fund Program and Office of Disease Prevention (ODP) sponsored a workshop on the Economics of Prevention. The goals of the workshop were to discuss the state of the research field, and identify gaps and opportunities to be addressed in future research.
On August 27 and 28, 2015, the National Institutes of Health (NIH) Health Economics Common Fund Program and Office of Disease Prevention (ODP) sponsored a workshop on the Economics of Prevention. The goals of the workshop were to discuss the state of the research field, and identify gaps and opportunities to be addressed in future research.
Read the Workshop Executive Summary.
Read the Workshop Agenda.
See a list of Workshop Attendees.
Download the full Workshop Report.
Personalized Health Care and Prevention Stakeholder Engagement Workshop
![]() On February 25, 2015, the National Institutes of Health (NIH) Health Economics Common Fund Program convened a workshop to engage a range of stakeholders in discussions about how NIH-funded research can enhance the role of personalized medicine in improving the efficiency and effectiveness of health care.
On February 25, 2015, the National Institutes of Health (NIH) Health Economics Common Fund Program convened a workshop to engage a range of stakeholders in discussions about how NIH-funded research can enhance the role of personalized medicine in improving the efficiency and effectiveness of health care.
The goal of the workshop was to facilitate dialogue among researchers, stakeholders, and NIH staff to (1) inform stakeholders of ongoing NIH-funded research initiatives and (2) help researchers focus on questions of critical value to stakeholders.
Read the Meeting Summary.
Senior Policy Researcher Dr. Chapin White (link is external) met with the Health Economics working group to discuss his research into "Designing Health Plan Networks to Steer Patients to Higher-value Providers." A summary of his remarks can be found here.
Study results from Health Economics-funded researcher Jody Sindelar suggest that for low-income individuals, a greater emphasis on the financial costs of smoking could be more effective to motivate quitting than health-related messages. Read the article summary...
Noted economist Martin F. Gaynor met with the Health Economics Working Group to discuss his thoughts about the current state of health care markets and areas for future research. A summary of his remarks can be found here.
The Health Economics Working Group partnered with the editors of the Forum for Health Economics & Policy to publish a special issue on the economics of personalized health care and prevention. The issue features an introduction by Working Group members Gregory Bloss (NIAAA) and John G. Haaga (NIA) and revised versions of the commissioned papers from five teleconference participants: Anirban Basu, Dana Goldman, Donald Kenkel, David Meltzer, and Kathryn Phillips.
The special issue can be accessed at: http://www.degruyter.com/view/j/fhep.2013.16.issue-2/issue-files/fhep.2013.16.issue-2.xml (link is external)
In 2012 the Health Economics Working Group held teleconferences with invited experts to discuss the state of the science concerning the determinants and consequences of personalized health care and to discuss medical technologies.
View the executive summaries here:
- Health Economics of Personalized Health Care and Prevention Teleconference
- Health Economics Teleconference: Diffusion of Medical Technology
Health Economics State Health Practice Database for Research Contract
The Health Economics Program of the Common Fund announced a contract to IMPAQ International, LLC and its subcontractors to design and create a State Health Practice Database for Research (SHPDR). This contract, supported after a competitive review of proposals, will meet the need for state-level data for research on a range of issues affecting health outcomes, in an environment in which health care delivery systems, health care financing, and health care technology are all changing rapidly. The resource resulting from this contract will be a core data set with numeric and categorical variables on health practices that can be used with statistical software, and can be linked with existing health and economic data for hypothesis-driven research. The need for this resource was discussed by experts at a 2010 NIH meeting and elaborated in responses from the scientific community to a Request for Information. Working Group members also consulted with Office of the Assistant Secretary of Planning and Evaluation and other HHS agencies to avoid duplication of efforts.
 Common Fund’s Health Economics Program Makes First Awards
Common Fund’s Health Economics Program Makes First Awards
 Common Fund Supports Meeting on Health Economics: NIH Research Priorities for Health Care Reform
Common Fund Supports Meeting on Health Economics: NIH Research Priorities for Health Care Reform