Science Highlights
- Cognitive Behavioral Therapy Can Reduce Chronic Pain and Pain-Related Disability
- Collaborative Care Intervention Can Reduce PTSD in Trauma Survivors
- LIRE Trial Publishes Primary Results
- PROVEN Trial Publishes Primary Results
- NIH Collaboratory to Serve as Coordinating Center for New Pragmatic Trials Addressing Opioid Crisis
- NIH Collaboratory Principal Investigator Receives National Award
- HCS Collaboratory Announces New Awards
- Antiseptic Bathing Reduces Infections in Hospital Patients
- STOP CRC Trial Demonstrates the Effectiveness of In-Home Colon Cancer Screening
- NIH Collaboratory ePCT Training Workshop Resources Now Available
- ICD Pieces Pragmatic Clinical Trial Begins Enrollment
- Collaboratory's Regulatory Ethics/Core Publishes Study on Doctors' viewpoints on Pragmatic Clinical Trials
- Ethical & Regulatory Issues of Pragmatic Clinical Trials Workshop
- Protocol Published for Collaboratory Pragmatic Clinical Trial to Improve Care for Patients with PTSD
- Collaboratory Pragmatic Clinical Trial to Reduce Hospital Acquired Infections Completes its Intervention Phase
- Collaboratory Publications on Research Ethics
- The Collaboratory's Health Care Systems Interactions Core Shares Lessons Learned & New Guidance
- How Pragmatic Is It? Applying the PRECIS Rating System to 5 Collaboratory Pragmatic Clinical Trials
- Study Design of LIRE Pragmatic Trial Published
- Findings from STOP CRC on Pragmatic Trial Recruitment
- Interview with Dr. Josephine Briggs on the Impact of HCS Research Collaboratory
- Collaboratory Researchers Find Poor Compliance with Clinical Trials Reporting Law
- Ethical and Regulatory Challenges for Pragmatic Cluster Randomized Trials
- "A guide to research partnerships for pragmatic clinical trials."
- Groundbreaking Suicide Study
- Large Data Networks to Support Public Health and Research
- STOP Colon Cancer Demonstration Project
- Electronic Health Records and Pragmatic Clinical Trials
- Improving Medical Research Studies through Participant Feedback
Cognitive Behavioral Therapy Can Reduce Chronic Pain and Pain-Related Disability
 The Collaborative Care for Chronic Pain in Primary Care (PPACT) study, an NIH Collaboratory Demonstration Project, published the primary results from their trial. The study was conducted under real-world conditions in Kaiser Permanente primary care clinics across three US regions (Georgia, Hawaii, and Northwest) to determine the effectiveness of a group-based cognitive behavioral therapy (CBT) intervention for chronic pain and functional impairment in patients receiving long-term opioid therapy. Participants were randomly assigned to an intervention or to standard care. The study showed that patients who participated in the intervention as part of their regular care for chronic pain showed improved function and reduced pain compared to standard treatment, but did not reduce their use of opioid medication. The CBT intervention consisted of (1) tailoring intervention goals to each patient (based on patients’ specific circumstances and preferences), and (2) twelve weekly group sessions focused on providing specific core skills (i.e., training in muscle relaxation techniques) and a yoga-based adaptive movement training. Learn more about this work: PPACT Study Finds Benefits of Cognitive Behavioral Therapy in Reducing Chronic Pain and Pain-Related Disability.
The Collaborative Care for Chronic Pain in Primary Care (PPACT) study, an NIH Collaboratory Demonstration Project, published the primary results from their trial. The study was conducted under real-world conditions in Kaiser Permanente primary care clinics across three US regions (Georgia, Hawaii, and Northwest) to determine the effectiveness of a group-based cognitive behavioral therapy (CBT) intervention for chronic pain and functional impairment in patients receiving long-term opioid therapy. Participants were randomly assigned to an intervention or to standard care. The study showed that patients who participated in the intervention as part of their regular care for chronic pain showed improved function and reduced pain compared to standard treatment, but did not reduce their use of opioid medication. The CBT intervention consisted of (1) tailoring intervention goals to each patient (based on patients’ specific circumstances and preferences), and (2) twelve weekly group sessions focused on providing specific core skills (i.e., training in muscle relaxation techniques) and a yoga-based adaptive movement training. Learn more about this work: PPACT Study Finds Benefits of Cognitive Behavioral Therapy in Reducing Chronic Pain and Pain-Related Disability.
Reference:
A Primary Care–Based Cognitive Behavioral Therapy Intervention for Long-Term Opioid Users With Chronic Pain. . DeBar L, Mayhew M, Benes L, Bonifay A, Deyo RA, Elder CR, Keefe FJ, Leo MC, McMullen C, Owen-Smith A, Smith DH, Trinacty CM, Vollmer WM. Ann Intern Med. 2021 Nov 2. doi: 10.7326/M21-1436.
Collaborative Care Intervention Can Reduce PTSD in Trauma Survivors
 The primary results of the Trauma Survivors Outcomes and Support (TSOS) trial, an NIH Collaboratory Demonstration Project, have been published. The study showed that a collaborative care intervention for injured patients at trauma centers can reduce symptoms of posttraumatic stress disorder (PTSD). The collaborative care consisted of evidence-based medication, cognitive behavioral therapy, and case management. Learn more about this work: TSOS Study Intervention Reduces PTSD Symptoms in Injured Patients at Level I Trauma Centers.
The primary results of the Trauma Survivors Outcomes and Support (TSOS) trial, an NIH Collaboratory Demonstration Project, have been published. The study showed that a collaborative care intervention for injured patients at trauma centers can reduce symptoms of posttraumatic stress disorder (PTSD). The collaborative care consisted of evidence-based medication, cognitive behavioral therapy, and case management. Learn more about this work: TSOS Study Intervention Reduces PTSD Symptoms in Injured Patients at Level I Trauma Centers.
Reference:
Stepped Collaborative Care Targeting Posttraumatic Stress Disorder Symptoms and Comorbidity for US Trauma Care Systems: A Randomized Clinical Trial. Zatzick D, Jurkovich G, Heagerty P, Russo J, Darnell D, Parker L, Roberts MK, Moodliar R, Engstrom A, Wang J, Bulger E, Whiteside L, Nehra D, Palinkas LA, Moloney K, Maier R. JAMA Surg. 2021 Mar 10. doi: 10.1001/jamasurg.2021.0131
 LIRE Trial Publishes Primary Results
LIRE Trial Publishes Primary Results
 Primary results have been published of the Lumbar Imaging With Reporting of Epidemiology (LIRE) trial, an NIH Collaboratory Demonstration Project. The study found that including additional information about the prevalence of common findings in spinal imaging reports has little impact on subsequent spine-related healthcare use, but may slightly reduce subsequent opioid prescriptions in a subset of patients.
Primary results have been published of the Lumbar Imaging With Reporting of Epidemiology (LIRE) trial, an NIH Collaboratory Demonstration Project. The study found that including additional information about the prevalence of common findings in spinal imaging reports has little impact on subsequent spine-related healthcare use, but may slightly reduce subsequent opioid prescriptions in a subset of patients.
Learn more on the NIH Collaboratory website and watch the November 8, 2019 Grand Rounds webinar for a presentation of the LIRE results: Lumbar Imaging with Reporting of Epidemiology: Initial Results and Some Lessons Learned (Jeffrey Jarvik, MD, MPH, Patrick Heagerty, PhD).
Reference:
The Effect of Including Benchmark Prevalence Data of Common Imaging Findings in Spine Image Reports on Health Care Utilization Among Adults Undergoing Spine Imaging: A Stepped-Wedge Randomized Clinical Trial. Jarvik JG, et al. JAMA Network Open. 2020 September 4. doi: 10.1001/jamanetworkopen.2020.15713.
 PROVEN Trial Publishes Primary Results
PROVEN Trial Publishes Primary Results
 The primary results of the Pragmatic Trial of Video Education in Nursing Homes (PROVEN), an NIH Collaboratory Demonstration Project, have been published. The study showed that the video education intervention did not have a marked effect on reducing the number of transfers from the nursing home to the hospital, highlighting the challenges of implementing new programs in nursing homes.
The primary results of the Pragmatic Trial of Video Education in Nursing Homes (PROVEN), an NIH Collaboratory Demonstration Project, have been published. The study showed that the video education intervention did not have a marked effect on reducing the number of transfers from the nursing home to the hospital, highlighting the challenges of implementing new programs in nursing homes.
Learn more on the NIH Collaboratory website and watch the June 12, 2020, Grand Rounds webinar for a presentation of the PROVEN results: A Cluster Randomized Pragmatic Trial of an Advance Care Planning Video Intervention in Long-Stay Nursing Home Residents: Main Findings from the PROVEN Trial (Susan Mitchell, MD, MPH).
Reference:
Advance Care Planning Video Intervention Among Long-Stay Nursing Home Residents: A Pragmatic Cluster Randomized Clinical Trial. Mitchell SL, Volandes AE, Gutman R, Gozalo PL, Ogarek JA, Loomer L, McCreedy EM, Zhai R, Mor V. JAMA Intern Med. 2020 July 6. doi: 10.1001/jamainternmed.2020.2366.
 NIH Collaboratory to Serve as Coordinating Center for New Pragmatic Trials Addressing Opioid Crisis
NIH Collaboratory to Serve as Coordinating Center for New Pragmatic Trials Addressing Opioid Crisis
The NIH Collaboratory Coordinating Center is serving as the Resource Coordinating Center for a new group of large-scale embedded pragmatic clinical trials (ePCTs) on pain management and reducing opioid prescribing. As part of the NIH Collaboratory, the Pragmatic and Implementation Studies for the Management of Pain to Reduce Opioid Prescribing (PRISM) Resource Coordinating Center will provide technical support and pragmatic trial expertise for the research that this program funds. Learn more on the NIH Collaboratory website.
 NIH Collaboratory Principal Investigator Receives National Award
NIH Collaboratory Principal Investigator Receives National Award
Congratulations to Dr. Greg Simon, PI of the Suicide Prevention Outreach Trial (SPOT), on receiving the American Foundation for Suicide Prevention Research Award for his contributions to suicide prevention! Learn more about this honor and Dr. Simon's research here.
 HCS Collaboratory Announces New Awards
HCS Collaboratory Announces New Awards
The NIH has announced five new awards that, with support from six NIH institutes, centers, and offices, will add additional pragmatic clinical trials projects to the HCS Collaboratory. More information can be found in the press release.
 Antiseptic Bathing Reduces Infections in Hospital Patients
Antiseptic Bathing Reduces Infections in Hospital Patients
 Hospital associated infections result in billions of dollars of healthcare costs and thousands of deaths every year. The Active Bathing to Eliminate Infection (ABATE) Trial, led by Dr. Susan Huang from the University of California, Irvine, collected real world data on bathing hospital patients with an antiseptic soap, chlorhexidine, during inpatient hospital stays. A previous study from Dr. Huang and colleagues showed that using this type of bathing as routine care in the intensive care unit (ICU) reduced bloodstream infections by up to 44 percent. Given those exciting results, the ABATE study was designed to test whether bathing with chlorhexidine as well as targeted use of a nasal antibiotic, mupirocin, would also reduce infections in non-ICU patients.
Hospital associated infections result in billions of dollars of healthcare costs and thousands of deaths every year. The Active Bathing to Eliminate Infection (ABATE) Trial, led by Dr. Susan Huang from the University of California, Irvine, collected real world data on bathing hospital patients with an antiseptic soap, chlorhexidine, during inpatient hospital stays. A previous study from Dr. Huang and colleagues showed that using this type of bathing as routine care in the intensive care unit (ICU) reduced bloodstream infections by up to 44 percent. Given those exciting results, the ABATE study was designed to test whether bathing with chlorhexidine as well as targeted use of a nasal antibiotic, mupirocin, would also reduce infections in non-ICU patients.
The ABATE study was conducted in 53 hospitals and compared routine bathing to bathing with chlorhexidine in over 330,000 non-ICU patients. In this large and diverse population of patients, there was not a statistically significant difference in the number of infections that occurred between the control and antiseptic bathing, suggesting that switching to this type of bathing as routine practice for all patients would not be an effective method for reducing infections. However, when considering only patients with a medical device, such as a central venous catheter or a lumbar drain, there was a reduction in bloodstream infections of over 30 percent when patients were assigned to the antiseptic bathing. Therefore, this bathing practice should be valuable for this higher risk subset of hospital patients. One of the health care systems in which this study took place already adopted this form of bathing for these patients because of the results from the ABATE Trial.
You can learn more about this study in the NIH press release that accompanied the study publication and by exploring the study page on the Collaboratory website.
Reference:
Chlorhexidine versus routine bathing to prevent multidrug-resistant organisms and all-cause bloodstream infections in general medical and surgical units (ABATE Infection trial): a cluster-randomised trial. Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Heim L, Gombosev A, Avery TR, Haffenreffer K, Shimelman L, Hayden MK, Weinstein RA, Spencer-Smith C, Kaganov RE, Murphy MV, Forehand T, Lankiewicz J, Coady MH, Portillo L, Sarup-Patel J, Jernigan JA, Perlin JB, Platt R, ABATE Infection trial team. Lancet. 2019 Mar 4. doi: 10.1016/S0140-6736(18)32593-5.
 STOP CRC Trial Demonstrates the Effectiveness of In-Home Colon Cancer Screening
STOP CRC Trial Demonstrates the Effectiveness of In-Home Colon Cancer Screening
 The HCS Research Collaboratory funded pragmatic clinical trial Strategies and Opportunities to Stop Colorectal Cancer in Priority Populations (STOP CRC) published the resultsof a program designed to test the effectiveness of mailing an in-home colon cancer screening test. This innovative program resulted in a nearly 4 percentage point increase in the colon cancer screening rate despite issues with getting clinics to fully implement the program.
The HCS Research Collaboratory funded pragmatic clinical trial Strategies and Opportunities to Stop Colorectal Cancer in Priority Populations (STOP CRC) published the resultsof a program designed to test the effectiveness of mailing an in-home colon cancer screening test. This innovative program resulted in a nearly 4 percentage point increase in the colon cancer screening rate despite issues with getting clinics to fully implement the program.
The trial was run in 26 Federally Qualified Health Centers (FQHCs) and resulted in over six thousand people receiving a screening kit in the mail. FQHCs serve a population that have an extremely low level of baseline screening for colon cancer and thus stand to benefit the most from this intervention. These clinics also tend to have limited time and resources, however those that were able to complete the mailings showed substantial increases in the number of people who were ultimately screened. These findings demonstrate the importance of doing a trial in real world setting, as even though the in-home testing for colon cancer is very effective, equal attention needs to be paid to generating strategies that allow effective implementation in a busy clinic environment.
You can read more about this study in a press release from Kaiser Permanente. The principal investigators for STOP CRC also recently talked about the results of this trial and the lessons learned from it in a recent NIH Collaboratory Grand Rounds presentation
Reference:
Effectiveness of a Mailed Colorectal Cancer Screening Outreach Program in Community Health Clinics: The STOP CRC Cluster Randomized Clinical Trial. Coronado GD, Petrik AF, Vollmer WM, Taplin SH, Keast EM, Fields S, Green BB. JAMA Intern Med. 2018 Aug 6. doi: 10.1001/jamainternmed.2018.3629.
 NIH Collaboratory ePCT Training Workshop Resources Now Available
NIH Collaboratory ePCT Training Workshop Resources Now Available
 The NIH Collaboratory held a pilot training workshop to teach lessons learned about the complexities of designing and conducting embedded pragmatic clinical trials (ePCTs) to mid- and senior-level investigators. The workshop was comprised of 10 topics across 2 days and included hands-on exercises and case studies from the Collaboratory Demonstration Projects illustrating the particulars of designing and conducting ePCTs and providing resources for planning and overcoming challenges. The slides and handouts from the workshop are now available for download on the Collaboratory website.
The NIH Collaboratory held a pilot training workshop to teach lessons learned about the complexities of designing and conducting embedded pragmatic clinical trials (ePCTs) to mid- and senior-level investigators. The workshop was comprised of 10 topics across 2 days and included hands-on exercises and case studies from the Collaboratory Demonstration Projects illustrating the particulars of designing and conducting ePCTs and providing resources for planning and overcoming challenges. The slides and handouts from the workshop are now available for download on the Collaboratory website.
 ICD Pieces Pragmatic Clinical Trial Begins Enrollment
ICD Pieces Pragmatic Clinical Trial Begins Enrollment
 ICD Pieces is a pragmatic clinical trial demonstration project supported by the Collaboratory. The trial will implement a new technology platform (Pieces) to enable use of electronic health record data to improve care for patients with chronic kidney disease and other associated conditions. Congratulations to the ICD Pieces Team on this important milestone! Learn more about the ICD Pieces trial.
ICD Pieces is a pragmatic clinical trial demonstration project supported by the Collaboratory. The trial will implement a new technology platform (Pieces) to enable use of electronic health record data to improve care for patients with chronic kidney disease and other associated conditions. Congratulations to the ICD Pieces Team on this important milestone! Learn more about the ICD Pieces trial.
 Collaboratory's Regulatory Ethics/Core Publishes Study on Doctors' viewpoints on Pragmatic Clinical Trials
Collaboratory's Regulatory Ethics/Core Publishes Study on Doctors' viewpoints on Pragmatic Clinical Trials
 The Collaboratory's Regulatory/Ethics Core found that while doctors are generally willing to participate in pragmatic clinical trials, “their support is predicated on several factors including expected benefits, minimization of time and workflow burdens, and physician engagement.” Read the article abstract.
The Collaboratory's Regulatory/Ethics Core found that while doctors are generally willing to participate in pragmatic clinical trials, “their support is predicated on several factors including expected benefits, minimization of time and workflow burdens, and physician engagement.” Read the article abstract.
Reference:
Physicians' perspectives regarding pragmatic clinical trials. Topazian R, Bollinger J, Weinfurt KP, Dvoskin R, Mathews D, Brelsford K, DeCamp M, Sugarman J. J Comp Eff Res. 2016 Aug;5(5):499-506. doi: 10.2217/cer-2016-0024. Epub 2016 Jul 15.
 Ethical & Regulatory Issues of Pragmatic Clinical Trials Workshop
Ethical & Regulatory Issues of Pragmatic Clinical Trials Workshop
> If you missed the May 10 Workshop, now you can watch the proceedings at your leisure. Watch the archived videocast. Read a brief blog post by Dr. Catherine Meyers of the NIH National Center for Complementary and Integrative Health (NCCIH) describing the workshop.
If you missed the May 10 Workshop, now you can watch the proceedings at your leisure. Watch the archived videocast. Read a brief blog post by Dr. Catherine Meyers of the NIH National Center for Complementary and Integrative Health (NCCIH) describing the workshop.
 Protocol Published for Collaboratory Pragmatic Clinical Trial to Improve Care for Patients with PTSD
Protocol Published for Collaboratory Pragmatic Clinical Trial to Improve Care for Patients with PTSD
 Researchers conducting the Trauma Survivors Outcomes and Support (TSOS) pragmatic clinical trial recently published the protocol for their trial in the journal Implementation Science. The TSOS trial is supported by the Health Care Systems Research Collaboratory as a demonstration project. It will take place at 24 US Level I trauma centers, and is designed to test screening and intervention strategies for patients with posttraumatic stress disorder (PTSD) and other associated conditions. Read the article abstract. Learn more about the TSOS trial.
Researchers conducting the Trauma Survivors Outcomes and Support (TSOS) pragmatic clinical trial recently published the protocol for their trial in the journal Implementation Science. The TSOS trial is supported by the Health Care Systems Research Collaboratory as a demonstration project. It will take place at 24 US Level I trauma centers, and is designed to test screening and intervention strategies for patients with posttraumatic stress disorder (PTSD) and other associated conditions. Read the article abstract. Learn more about the TSOS trial.
Reference:
An effectiveness-implementation hybrid trial study protocol targeting posttraumatic stress disorder and comorbidity. Zatzick DF, Russo J, Darnell D, Chambers DA, Palinkas L, Van Eaton E, Wang J, Ingraham LM, Guiney R, Heagerty P, Comstock B, Whiteside LK, Jurkovich G. Implement Sci. 2016 Apr 30;11(1):58.
 Collaboratory Pragmatic Clinical Trial to Reduce Hospital Acquired Infections Completes its Intervention Phase
Collaboratory Pragmatic Clinical Trial to Reduce Hospital Acquired Infections Completes its Intervention Phase
The Active Bathing to Eliminate (ABATE) Infection trial has completed its intervention phase - the part of the trial where patients are treated and data are collected. The large-scale trial was designed to assess an approach for reducing multidrug-resistant organisms and hospital-associated infections in nearly 200 non-critical care hospital units across the United States. Now the study team heads into the data cleaning and analysis phase. Read more about this major milestone for the ABATE trial.
Image at right: Electron microscope image of Methicillin-resistant S. aureus (MRSA) bacteria. Image courtesy of the Centers for Disease Control and Prevention (CDC).
 Collaboratory Publications on Research Ethics
Collaboratory Publications on Research Ethics
 Members of the Collaboratory's Regulatory/Ethics core group recently published three articles in the American Journal of Bioethics. The articles address various questions related to research on medical practices including: shared medical decision making, patient viewpoints on research concerning medical practice, and the ethics of research conducted in usual health care settings. The second two publications are part of a special issue of the journal that broadly addresses the "Ethics of Research in Usual Care Settings."
Members of the Collaboratory's Regulatory/Ethics core group recently published three articles in the American Journal of Bioethics. The articles address various questions related to research on medical practices including: shared medical decision making, patient viewpoints on research concerning medical practice, and the ethics of research conducted in usual health care settings. The second two publications are part of a special issue of the journal that broadly addresses the "Ethics of Research in Usual Care Settings."
References (Note: full text may require institutional access)
- Is Shared Decision Making an Appropriate Analytic Frame for Research on Medical Practices? Sugarman, J. Am J Bioeth. 2015;15(9):18-20.
- Patients' views concerning research on medical practices: Implications for consent Kevin P. Weinfurt, Juli M. Bollinger, Kathleen M. Brelsford, Travis J. Crayton, Rachel J. Topazian, Nancy E. Kass, Laura M. Beskow & Jeremy Sugarman. Am J Bioeth. 2016;0(0):1-16.
- Ethics of Research in Usual Care Settings: Data on Point Sugarman, J. Am J Bioeth. Accepted author version posted online: 10 Feb 2016. DOI:10.1080/23294515.2016.1152104.
 Health Care Systems Interactions Core Shares Lessons Learned & New Guidance
Health Care Systems Interactions Core Shares Lessons Learned & New Guidance
Two documents are now available describing lessons learned from the Pragmatic Clinical Trials (PCTs) supported by the Collaboratory and how training for PCTs differs from typical clinical research studies. Read a short description and find links to the documents.
 How Pragmatic Is It? Applying the PRECIS Rating System to 5 Collaboratory Pragmatic Clinical Trials
How Pragmatic Is It? Applying the PRECIS Rating System to 5 Collaboratory Pragmatic Clinical Trials
An article published in the journal Trials describes how the Pragmatic – Explanatory Continuum Indicator Summary, or PRECIS, rating system can be applied to clinical trial designs to see where a given study sits on the spectrum of typical explanatory trials versus pragmatic clinical trials. The authors apply the rating system to 5 of the pragmatic trial demonstration projects supported by the Collaboratory. Read a brief summary of the article in plain language.
Reference:
Use of PRECIS ratings in the National Institutes of Health (NIH) Health Care Systems Research Collaboratory. Johnson KE, Neta G, Dember LM, Coronado GD, Suls J, Chambers DA, Rundell S, Smith DH, Liu B, Taplin S, Stoney CM, Farrell MM, Glasgow RE. Trials: 17(1):32. January 16, 2016.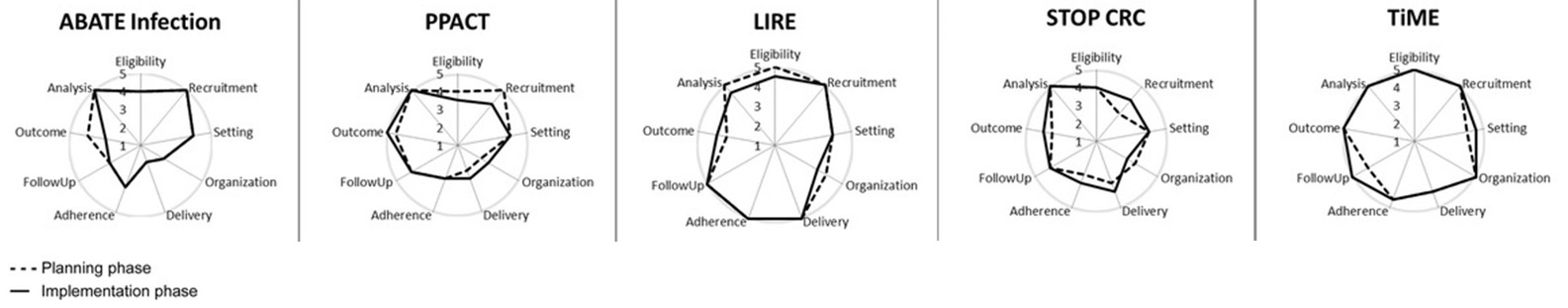
 Study Design of LIRE Pragmatic Trial Published
Study Design of LIRE Pragmatic Trial Published
 Dr. Jerry Jarvik and colleagues have published an article in Contemporary Clinical Trials describing the design of the Lumbar Imaging With Reporting of Epidemiology (LIRE) trial. The trial is being supported by the HCS Research Collaboratory as a demonstration project. Read a brief summary of the article in plain language. Read the original article abstract.
Dr. Jerry Jarvik and colleagues have published an article in Contemporary Clinical Trials describing the design of the Lumbar Imaging With Reporting of Epidemiology (LIRE) trial. The trial is being supported by the HCS Research Collaboratory as a demonstration project. Read a brief summary of the article in plain language. Read the original article abstract.
Reference:
Lumbar Imaging With Reporting Of Epidemiology (LIRE)-Protocol for a pragmatic cluster randomized trial. Jarvik JG, Comstock BA, James KT, Avins AL, Bresnahan BW, Deyo RA, Luetmer PH, Friedly JL, Meier EN, Cherkin DC, Gold LS, Rundell SD, Halabi SS, Kallmes DF, Tan KW, Turner JA, Kessler LG, Lavallee DC, Stephens KA, Heagerty PJ. Contemporary Clinical Trials. doi: 10.1016/j.cct.2015.10.003. [Epub ahead of print] October 19, 2015.
 Findings from STOP CRC on Pragmatic Trial Recruitment
Findings from STOP CRC on Pragmatic Trial Recruitment
Drs. Coronado and Green, lead researchers of the Strategies and Opportunities to Stop Colorectal Cancer in Priority Populations (STOP CRC) pragmatic trial demonstration project, and colleagues have published an article in Clinical Trials describing the challenges of recruiting participants into large, multi-site pragmatic clinical trials—particularly at the health system level. Read a brief summary of the article in plain language. Read the original article abstract.
Reference:
Recruiting community health centers into pragmatic research: Findings from STOP CRC. Coronado GD, Retecki S, Schneider J, Taplin SH, Burdick T, Green BB. Clinical Trials. 2015 Sep 29. pii: 1740774515608122. [Epub ahead of print].
 Interview with Dr. Josephine Briggs on the Impact of HCS Research Collaboratory
Interview with Dr. Josephine Briggs on the Impact of HCS Research Collaboratory
Click on the image at right to watch an interview with the Director of the NIH National Center for Complementary and Integrative Health (NCCIH) as she describes the impact of the HCS Research Collaboratory and its collaborators.
 Collaboratory Researchers Find Poor Compliance with Clinical Trials Reporting Law
Collaboratory Researchers Find Poor Compliance with Clinical Trials Reporting Law
An analysis of data from the ClinicalTrials.gov website shows that despite federal laws requiring the public reporting of results from clinical trials, most research sponsors fail to do so in a timely fashion — or, in many cases, at all. The study, published in the New England Journal of Medicine, was conducted by researchers supported by the NIH Collaboratory and the Clinical Trials Transformation Initiative (CTTI). A brief description of the findings is available in the Collaboratory's Living Textbook "Rethinking Clinical Trials." The original research article was published in The New England Journal of Medicine. The article abstract is freely available.
 Ethical and Regulatory Challenges for Pragmatic Cluster Randomized Trials
Ethical and Regulatory Challenges for Pragmatic Cluster Randomized Trials
A report by researchers from the NIH Collaboratory explores some of the challenges facing physicians, scientists, and patient groups who are working to develop innovative methods for performing clinical trials. A brief description of the report is available in the Collaboratory's Living Textbook "Rethinking Clinical Trials." The original research article was published in the journal Clinical Trials. The article abstract is freely available.
 "A guide to research partnerships for pragmatic clinical trials."
"A guide to research partnerships for pragmatic clinical trials."
The HCS Research Collaboratory's Systems Interactions Core published a paper that highlights multiple lessons about building strong research partnerships between health researchers and healthcare systems.
Read the full article in The BMJ.
Supplemental Material is available in the Collaboratory's Living Textbook.
 Groundbreaking Suicide Study
Groundbreaking Suicide Study
An HCS Research Collaboratory Demonstration Project will help researchers learn more about ways to treat people experiencing suicidal thoughts. Nearly 20,000 patients will be able to participate in a trial that draws from other successful interventions for depression and suicide. One of the treatments being tested was developed with the help of other patients.
Read the Science Update from the National Institute of Mental Health about the "Pragmatic Trial of Population-Based Programs to Prevent Suicide Attempt" Demonstration Project.
Get Detailed Information on the Demonstration Project from the HCS Research Collaboratory website.
 Large Data Networks to Support Public Health and Research
Large Data Networks to Support Public Health and Research
 HCS Research Collaboratory grantee, Dr. Richard Platt, and his colleagues published an article in Health Affairs profiling four large health data networks. The networks are “examples of the first stage in the development of a shared national big-data resource that leverages the investments of many agencies and organizations for the benefit of multiple networks and users.” Read the original article abstract (full text requires subscription).
HCS Research Collaboratory grantee, Dr. Richard Platt, and his colleagues published an article in Health Affairs profiling four large health data networks. The networks are “examples of the first stage in the development of a shared national big-data resource that leverages the investments of many agencies and organizations for the benefit of multiple networks and users.” Read the original article abstract (full text requires subscription).
Reference:
Four Health Data Networks Illustrate the Potential for a Shared National Multipurpose Big-Data Network. Curtis LH, Brown J, Platt R. Health Affairs. July 2014, vol. 33, no. 7, 1178-1186.
 STOP Colon Cancer Demonstration Project
STOP Colon Cancer Demonstration Project
This HCS Collaboratory Demonstration Project learned valuable lessons from a pilot study about promoting cancer screening among minority and low-income populations.
Read the National Public Radio "Shots Blog" post about the project.
Click on the image at right to watch a fun & educational video about the project.
Read the original research article in BMC Cancer.
 Electronic Health Records and Pragmatic Clinical Trials
Electronic Health Records and Pragmatic Clinical Trials
In an article in the Journal of the American Medical Informatics Association the HCS Research Collaboratory describes the challenges and opportunities faced in using electronic health records when conducting pragmatic clinical trials.
Read a brief description of the article from the HCS Collaboratory here.
 Improving Medical Research Studies through Participant Feedback
Improving Medical Research Studies through Participant Feedback
HCS Research Collaboratory steering committee chairman Barry Coller, M.D., published an article in the New England Journal of Medicine on how medical research participants’ experiences could be used to improve the planning process for medical research studies.





